Crystal Structures of TbCatB and rhodesain, potential chemotherapeutic targets and major cysteine proteases of Trypanosoma brucei
- PMID: 20544024
- PMCID: PMC2882330
- DOI: 10.1371/journal.pntd.0000701
Crystal Structures of TbCatB and rhodesain, potential chemotherapeutic targets and major cysteine proteases of Trypanosoma brucei
Abstract
Background: Trypanosoma brucei is the etiological agent of Human African Trypanosomiasis, an endemic parasitic disease of sub-Saharan Africa. TbCatB and rhodesain are the sole Clan CA papain-like cysteine proteases produced by the parasite during infection of the mammalian host and are implicated in the progression of disease. Of considerable interest is the exploration of these two enzymes as targets for cysteine protease inhibitors that are effective against T. brucei.
Methods and findings: We have determined, by X-ray crystallography, the first reported structure of TbCatB in complex with the cathepsin B selective inhibitor CA074. In addition we report the structure of rhodesain in complex with the vinyl-sulfone K11002.
Conclusions: The mature domain of our TbCat*CA074 structure contains unique features for a cathepsin B-like enzyme including an elongated N-terminus extending 16 residues past the predicted maturation cleavage site. N-terminal Edman sequencing reveals an even longer extension than is observed amongst the ordered portions of the crystal structure. The TbCat*CA074 structure confirms that the occluding loop, which is an essential part of the substrate-binding site, creates a larger prime side pocket in the active site cleft than is found in mammalian cathepsin B-small molecule structures. Our data further highlight enhanced flexibility in the occluding loop main chain and structural deviations from mammalian cathepsin B enzymes that may affect activity and inhibitor design. Comparisons with the rhodesain*K11002 structure highlight key differences that may impact the design of cysteine protease inhibitors as anti-trypanosomal drugs.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
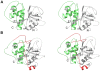


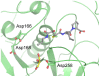


Similar articles
-
The Inhibition of Cysteine Proteases Rhodesain and TbCatB: A Valuable Approach to Treat Human African Trypanosomiasis.Mini Rev Med Chem. 2016;16(17):1374-1391. doi: 10.2174/1389557515666160509125243. Mini Rev Med Chem. 2016. PMID: 27156518 Review.
-
A cathepsin B-like protease is required for host protein degradation in Trypanosoma brucei.J Biol Chem. 2004 Nov 12;279(46):48426-33. doi: 10.1074/jbc.M402470200. Epub 2004 Aug 23. J Biol Chem. 2004. PMID: 15326171
-
Active site mapping, biochemical properties and subcellular localization of rhodesain, the major cysteine protease of Trypanosoma brucei rhodesiense.Mol Biochem Parasitol. 2001 Nov;118(1):61-73. doi: 10.1016/s0166-6851(01)00368-1. Mol Biochem Parasitol. 2001. PMID: 11704274
-
Vinyl sulfones as antiparasitic agents and a structural basis for drug design.J Biol Chem. 2009 Sep 18;284(38):25697-703. doi: 10.1074/jbc.M109.014340. Epub 2009 Jul 20. J Biol Chem. 2009. PMID: 19620707 Free PMC article.
-
Update on relevant trypanosome peptidases: Validated targets and future challenges.Biochim Biophys Acta Proteins Proteom. 2021 Feb;1869(2):140577. doi: 10.1016/j.bbapap.2020.140577. Epub 2020 Nov 30. Biochim Biophys Acta Proteins Proteom. 2021. PMID: 33271348 Review.
Cited by
-
Development of Novel Peptidyl Nitriles Targeting Rhodesain and Falcipain-2 for the Treatment of Sleeping Sickness and Malaria.Int J Mol Sci. 2024 Apr 17;25(8):4410. doi: 10.3390/ijms25084410. Int J Mol Sci. 2024. PMID: 38673995 Free PMC article.
-
Identification of plakortide E from the Caribbean sponge Plakortis halichondroides as a trypanocidal protease inhibitor using bioactivity-guided fractionation.Mar Drugs. 2014 May 2;12(5):2614-22. doi: 10.3390/md12052614. Mar Drugs. 2014. PMID: 24798927 Free PMC article.
-
Development of Urea-Bond-Containing Michael Acceptors as Antitrypanosomal Agents Targeting Rhodesain.ACS Med Chem Lett. 2022 Jun 30;13(7):1083-1090. doi: 10.1021/acsmedchemlett.2c00084. eCollection 2022 Jul 14. ACS Med Chem Lett. 2022. PMID: 35859868 Free PMC article.
-
A global comparison of the human and T. brucei degradomes gives insights about possible parasite drug targets.PLoS Negl Trop Dis. 2012;6(12):e1942. doi: 10.1371/journal.pntd.0001942. Epub 2012 Dec 6. PLoS Negl Trop Dis. 2012. PMID: 23236535 Free PMC article.
-
Drug targets in Leishmania.J Parasit Dis. 2010 Apr;34(1):1-13. doi: 10.1007/s12639-010-0006-3. Epub 2010 Oct 8. J Parasit Dis. 2010. PMID: 21526026 Free PMC article.
References
-
- Cox FE. History of sleeping sickness (African trypanosomiasis). Infect Dis Clin North Am. 2004;18:231–245. - PubMed
-
- Jannin J, Cattand P. Treatment and control of human African trypanosomiasis. Curr Opin Infect Dis. 2004;17:565–571. - PubMed
-
- Kaare MT, Picozzi K, Mlengeya T, Fevre EM, Mellau LS, et al. Sleeping sickness–a re-emerging disease in the Serengeti? Travel Med Infect Dis. 2007;5:117–124. - PubMed
-
- Burri C, Brun R. Eflornithine for the treatment of human African trypanosomiasis. Parasitol Res. 2003;90(Supp 1):S49–52. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

