GM-CSF increases mucosal and systemic immunogenicity of an H1N1 influenza DNA vaccine administered into the epidermis of non-human primates
- PMID: 20544035
- PMCID: PMC2882341
- DOI: 10.1371/journal.pone.0011021
GM-CSF increases mucosal and systemic immunogenicity of an H1N1 influenza DNA vaccine administered into the epidermis of non-human primates
Abstract
Background: The recent H5N1 avian and H1N1 swine-origin influenza virus outbreaks reaffirm that the threat of a world-wide influenza pandemic is both real and ever-present. Vaccination is still considered the best strategy for protection against influenza virus infection but a significant challenge is to identify new vaccine approaches that offer accelerated production, broader protection against drifted and shifted strains, and the capacity to elicit anti-viral immune responses in the respiratory tract at the site of viral entry. As a safe alternative to live attenuated vaccines, the mucosal and systemic immunogenicity of an H1N1 influenza (A/New Caledonia/20/99) HA DNA vaccine administered by particle-mediated epidermal delivery (PMED or gene gun) was analyzed in rhesus macaques.
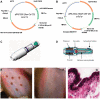
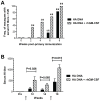
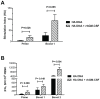
Methodology/principal findings: Macaques were immunized at weeks 0, 8, and 16 using a disposable single-shot particle-mediated delivery device designed for clinical use that delivers plasmid DNA directly into cells of the epidermis. Significant levels of hemagglutination inhibiting (HI) antibodies and cytokine-secreting HA-specific T cells were observed in the periphery of macaques following 1-3 doses of the PMED HA DNA vaccine. In addition, HA DNA vaccination induced detectable levels of HA-specific mucosal antibodies and T cells in the lung and gut-associated lymphoid tissues of vaccinated macaques. Importantly, co-delivery of a DNA encoding the rhesus macaque GM-CSF gene was found to significantly enhance both the systemic and mucosal immunogenicity of the HA DNA vaccine.
Conclusions/significance: These results provide strong support for the development of a particle-mediated epidermal DNA vaccine for protection against respiratory pathogens such as influenza and demonstrate, for the first time, the ability of skin-delivered GM-CSF to serve as an effective mucosal adjuvant for vaccine induction of immune responses in the gut and respiratory tract.
Conflict of interest statement
Figures
Similar articles
-
Adenovirus and mRNA vaccines as well as mucosal boosting improve protective efficacy against influenza virus challenge in macaques.Sci Transl Med. 2025 Jul 9;17(806):eadu7646. doi: 10.1126/scitranslmed.adu7646. Epub 2025 Jul 9. Sci Transl Med. 2025. PMID: 40632835
-
Protective immunity against influenza H5N1 virus challenge in mice by intranasal co-administration of baculovirus surface-displayed HA and recombinant CTB as an adjuvant.Virology. 2008 Oct 25;380(2):412-20. doi: 10.1016/j.virol.2008.08.002. Epub 2008 Sep 10. Virology. 2008. PMID: 18786689
-
Immunization with a low dose of hemagglutinin-encoding plasmid protects against 2009 H1N1 pandemic influenza virus in mice.J Virol Methods. 2011 May;173(2):314-9. doi: 10.1016/j.jviromet.2011.03.001. Epub 2011 Mar 12. J Virol Methods. 2011. PMID: 21392537
-
Prospects for developing an effective particle-mediated DNA vaccine against influenza.Expert Rev Vaccines. 2009 Sep;8(9):1205-20. doi: 10.1586/erv.09.82. Expert Rev Vaccines. 2009. PMID: 19722894 Review.
-
DNA Vaccine Delivery and Improved Immunogenicity.Curr Issues Mol Biol. 2017;22:129-138. doi: 10.21775/cimb.022.129. Epub 2016 Nov 10. Curr Issues Mol Biol. 2017. PMID: 27831541 Review.
Cited by
-
Modulation of SIV and HIV DNA vaccine immunity by Fas-FasL signaling.Viruses. 2015 Mar 23;7(3):1429-53. doi: 10.3390/v7031429. Viruses. 2015. PMID: 25807052 Free PMC article.
-
Therapeutic DNA vaccine induces broad T cell responses in the gut and sustained protection from viral rebound and AIDS in SIV-infected rhesus macaques.PLoS One. 2012;7(3):e33715. doi: 10.1371/journal.pone.0033715. Epub 2012 Mar 19. PLoS One. 2012. PMID: 22442716 Free PMC article.
-
Fusion of antigen to a dendritic cell targeting chemokine combined with adjuvant yields a malaria DNA vaccine with enhanced protective capabilities.PLoS One. 2014 Mar 5;9(3):e90413. doi: 10.1371/journal.pone.0090413. eCollection 2014. PLoS One. 2014. PMID: 24599116 Free PMC article.
-
Designing a novel mRNA vaccine against SARS-CoV-2: An immunoinformatics approach.Int J Biol Macromol. 2020 Nov 1;162:820-837. doi: 10.1016/j.ijbiomac.2020.06.213. Epub 2020 Jun 26. Int J Biol Macromol. 2020. PMID: 32599237 Free PMC article.
-
Intranasal administration of cationic liposomes enhanced granulocyte-macrophage colony-stimulating factor expression and this expression is dispensable for mucosal adjuvant activity.BMC Res Notes. 2018 Jul 13;11(1):472. doi: 10.1186/s13104-018-3591-3. BMC Res Notes. 2018. PMID: 30005702 Free PMC article.
References
-
- Epstein SL, Lo CY, Misplon JA, Lawson CM, Hendrickson BA, et al. Mechanisms of heterosubtypic immunity to lethal influenza A virus infection in fully immunocompetent, T cell-depleted, beta2-microglobulin-deficient, and J chain-deficient mice. J Immunol. 1997;158:1222–1230. - PubMed
-
- Lo CY, Wu Z, Misplon JA, Price GE, Pappas C, et al. Comparison of vaccines for induction of heterosubtypic immunity to influenza A virus: cold-adapted vaccine versus DNA prime-adenovirus boost strategies. Vaccine. 2008;26:2062–2072. - PubMed
-
- Mitchell JA, Green TD, Bright RA, Ross TM. Induction of heterosubtypic immunity to influenza A virus using a DNA vaccine expressing hemagglutinin-C3d fusion proteins. Vaccine. 2003;21:902–914. - PubMed
-
- Price GE, Soboleski MR, Lo CY, Misplon JA, Pappas C, et al. Vaccination focusing immunity on conserved antigens protects mice and ferrets against virulent H1N1 and H5N1 influenza A viruses. Vaccine. 2009;27:6512–6521. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

