Intrinsic disorder and protein multibinding in domain, terminal, and linker regions
- PMID: 20544079
- PMCID: PMC2955455
- DOI: 10.1039/c005144f
Intrinsic disorder and protein multibinding in domain, terminal, and linker regions
Abstract
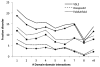
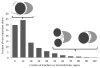
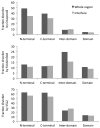
Intrinsic disorder is believed to contribute to the ability of some proteins to interact with multiple partners which is important for protein functional promiscuity and regulation of the cross-talk between pathways. To better understand the mechanisms of molecular recognition through disordered regions, here, we systematically investigate the coupling between disorder and binding within domain families in a structure interaction network and in terminal and inter-domain linker regions. We showed that the canonical domain-domain interaction model should take into account contributions of N- and C-termini and inter-domain linkers, which may form all or part of the binding interfaces. For the majority of proteins, binding interfaces on domain and terminal regions were predicted to be less disordered than non-interface regions. Analysis of all domain families revealed several exceptions, such as kinases, DNA/RNA binding proteins, certain enzymes, and regulatory proteins, which are candidates for disorder-to-order transitions that can occur upon binding. Domain interfaces that bind single or multiple partners do not exhibit significant difference in disorder content if normalized by the number of interactions. In general, protein families with more diverse interactions exhibit less average disorder over all members of the family. Our results shed light on recent controversies regarding the relationship between disorder and binding of multiple partners at common interfaces. In particular, they support the hypothesis that protein domains with many interacting partners should have a pleiotropic effect on functional pathways and consequently might be more constrained in evolution.
Figures
References
-
- Nobeli I, Favia AD, Thornton JM. Nat Biotechnol. 2009;27:157–167. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

