Allosteric effects of the antipsychotic drug trifluoperazine on the energetics of calcium binding by calmodulin
- PMID: 20544963
- PMCID: PMC2913171
- DOI: 10.1002/prot.22739
Allosteric effects of the antipsychotic drug trifluoperazine on the energetics of calcium binding by calmodulin
Abstract
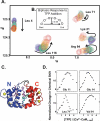
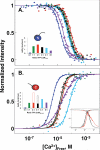
Trifluoperazine (TFP; Stelazine) is an antagonist of calmodulin (CaM), an essential regulator of calcium-dependent signal transduction. Reports differ regarding whether, or where, TFP binds to apo CaM. Three crystallographic structures (1CTR, 1A29, and 1LIN) show TFP bound to (Ca(2+))(4)-CaM in ratios of 1, 2, or 4 TFP per CaM. In all of these, CaM domains adopt the "open" conformation seen in CaM-kinase complexes having increased calcium affinity. Most reports suggest TFP also increases calcium affinity of CaM. To compare TFP binding to apo CaM and (Ca(2+))(4)-CaM and explore differential effects on the N- and C-domains of CaM, stoichiometric TFP titrations of CaM were monitored by (15)N-HSQC NMR. Two TFP bound to apo CaM, whereas four bound to (Ca(2+))(4)-CaM. In both cases, the preferred site was in the C-domain. During the titrations, biphasic responses for some resonances suggested intersite interactions. TFP-binding sites in apo CaM appeared distinct from those in (Ca(2+))(4)-CaM. In equilibrium calcium titrations at defined ratios of TFP:CaM, TFP reduced calcium affinity at most levels tested; this is similar to the effect of many IQ-motifs on CaM. However, at the highest level tested, TFP raised the calcium affinity of the N-domain of CaM. A model of conformational switching is proposed to explain how TFP can exert opposing allosteric effects on calcium affinity by binding to different sites in the "closed," "semi-open," and "open" domains of CaM. In physiological processes, apo CaM, as well as (Ca(2+))(4)-CaM, needs to be considered a potential target of drug action.
(c) 2010 Wiley-Liss, Inc.
Figures






References
-
- Hidaka H, Ishikawa T. Molecular pharmacology of calmodulin pathways in the cell functions. Cell Calcium. 1992;13:465–472. - PubMed
-
- Newman RA, Van Scyoc WS, Sorensen BR, Jaren OR, Shea MA. Interdomain coopertivity of calmodulin to melittin preferentially increases calcium affinity of sites I and II. Proteins: Structure, Function, and Bioinformatics. 2008;71(4):1792–1812. - PubMed
-
- Sorensen BR, Faga LA, Hultman R, Shea MA. Interdomain linker increases thermostability and decreases calcium affinity of calmodulin N-domain. Biochemistry. 2002;41(1):15–20. - PubMed
-
- Sorensen BR, Shea MA. Interactions between domains of apo calmodulin alter calcium binding and stability. Biochemistry. 1998;37:4244–4253. - PubMed
-
- Zhang M, Tanaka T, Ikura M. Calcium-induced conformational transition revealed by the solution structure of apo calmodulin. Nature Struct Biol. 1995;2:758–767. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

