Spectroscopic and mechanistic investigations of dehaloperoxidase B from Amphitrite ornata
- PMID: 20545299
- PMCID: PMC2921985
- DOI: 10.1021/bi100407v
Spectroscopic and mechanistic investigations of dehaloperoxidase B from Amphitrite ornata
Abstract
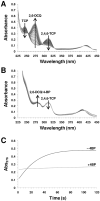
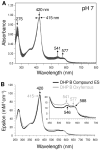
Dehaloperoxidase (DHP) from the terebellid polychaete Amphitrite ornata is a bifunctional enzyme that possesses both hemoglobin and peroxidase activities. Of the two DHP isoenzymes identified to date, much of the recent focus has been on DHP A, whereas very little is known pertaining to the activity, substrate specificity, mechanism of function, or spectroscopic properties of DHP B. Herein, we report the recombinant expression and purification of DHP B, as well as the details of our investigations into its catalytic cycle using biochemical assays, stopped-flow UV-visible, resonance Raman, and rapid freeze-quench electron paramagnetic resonance spectroscopies, and spectroelectrochemistry. Our experimental design reveals mechanistic insights and kinetic descriptions of the dehaloperoxidase mechanism which have not been previously reported for isoenzyme A. Namely, we demonstrate a novel reaction pathway in which the products of the oxidative dehalogenation of trihalophenols (dihaloquinones) are themselves capable of inducing formation of oxyferrous DHP B, and an updated catalytic cycle for DHP is proposed. We further demonstrate that, unlike the traditional monofunctional peroxidases, the oxyferrous state in DHP is a peroxidase-competent starting species, which suggests that the ferric oxidation state may not be an obligatory starting point for the enzyme. The data presented herein provide a link between the peroxidase and oxygen transport activities which furthers our understanding of how this bifunctional enzyme is able to unite its two inherent functions in one system.
Figures







References
-
- Weber RE, Mangum C, Steinman H, Bonaventura C, Sullivan B, Bonaventura J. Hemoglobins of two terebellid polychaetes: Enoplobranchus sanguineus and Amphitrite ornata. Comp Biochem Physiol A Comp Physiol. 1977;56:179–187. - PubMed
-
- Chen YP, Woodin SA, Lincoln DE, Lovell CR. An unusual dehalogenating peroxidase from the marine terebellid polychaete Amphitrite ornata. J Biol Chem. 1996;271:4609–4612. - PubMed
-
- Weber RE, Vinogradov SN. Nonvertebrate hemoglobins: functions and molecular adaptations. Physiol Rev. 2001;81:569–628. - PubMed
-
- Hardison R. Hemoglobins from bacteria to man: evolution of different patterns of gene expression. J Exp Biol. 1998;201:1099–1117. - PubMed
-
- Bailly X, Chabasse C, Hourdez S, Dewilde S, Martial S, Moens L, Zal F. Globin gene family evolution and functional diversification in annelids. Febs J. 2007;274:2641–2652. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

