The high affinity iron permease is a key virulence factor required for Rhizopus oryzae pathogenesis
- PMID: 20545847
- PMCID: PMC2909342
- DOI: 10.1111/j.1365-2958.2010.07234.x
The high affinity iron permease is a key virulence factor required for Rhizopus oryzae pathogenesis
Abstract
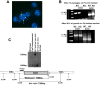
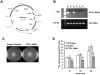
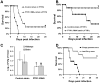
Rhizopus oryzae is the most common cause of mucormycosis, an angioinvasive fungal infection that causes more then 50% mortality rate despite first-line therapy. Clinical and animal model data clearly demonstrate that the presence of elevated available serum iron predisposes the host to mucormycosis. The high affinity iron permease gene (FTR1) is required for R. oryzae iron transport in iron-depleted environments. Here we demonstrate that FTR1 is required for full virulence of R. oryzae in mice. We show that FTR1 is expressed during infection in diabetic ketoacidosis (DKA) mice. In addition, we disrupted FTR1 by double cross-over homologous recombination, but multinucleated R. oryzae could not be forced to segregate to a homokaryotic null allele. Nevertheless, a reduction of the relative copy number of FTR1 and inhibition of FTR1 expression by RNAi compromised the ability of R. oryzae to acquire iron in vitro and reduced its virulence in DKA mice. Importantly, passive immunization with anti-Ftr1p immune sera protected DKA mice from infection with R. oryzae. Thus, FTR1 is a virulence factor for R. oryzae, and anti-Ftr1p passive immunotherapy deserves further evaluation as a strategy to improve outcomes of deadly mucormycosis.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures




References
-
- Artis WM, Fountain JA, Delcher HK, Jones HE. A mechanism of susceptibility to mucormycosis in diabetic ketoacidosis: transferrin and iron availability. Diabetes. 1982;31:1109–1114. - PubMed
-
- Boelaert JR, Van Cutsem J, de Locht M, Schneider YJ, Crichton RR. Deferoxamine augments growth and pathogenicity of Rhizopus, while hydroxypyridinone chelators have no effect. Kidney International. 1994;45:667–671. - PubMed
-
- Boelaert JR, van Roost GF, Vergauwe PL, Verbanck JJ, de Vroey C, Segaert MF. The role of desferrioxamine in dialysis-associated mucormycosis: report of three cases and review of the literature. Clinical Nephrology. 1988;29:261–266. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

