Identification of protein stability determinants in chloroplasts
- PMID: 20545891
- PMCID: PMC2988409
- DOI: 10.1111/j.1365-313X.2010.04268.x
Identification of protein stability determinants in chloroplasts
Abstract
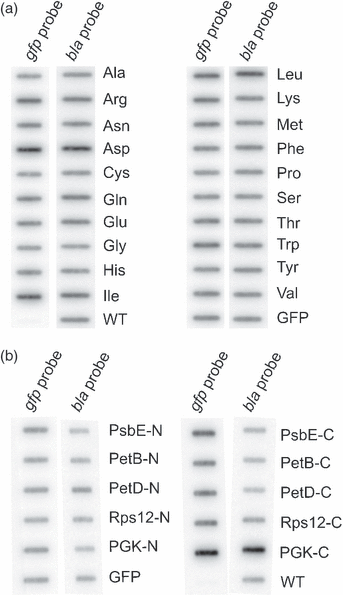
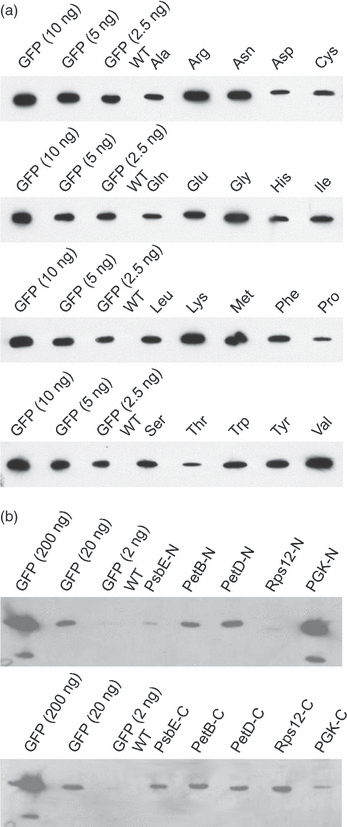
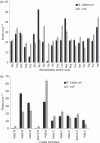
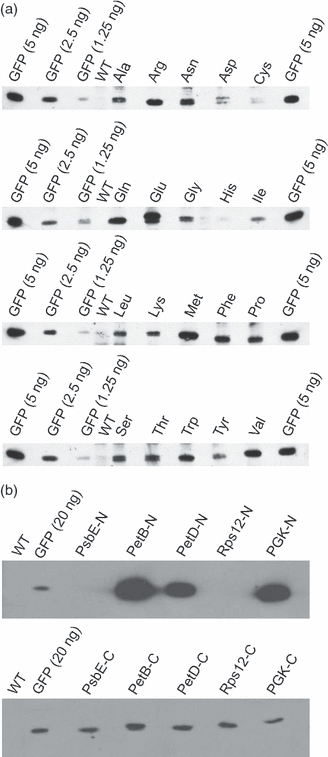
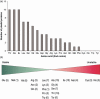
Although chloroplast protein stability has long been recognised as a major level of post-translational regulation in photosynthesis and gene expression, the factors determining protein stability in plastids are largely unknown. Here, we have identified stability determinants in vivo by producing plants with transgenic chloroplasts that express a reporter protein whose N- and C-termini were systematically modified. We found that major stability determinants are located in the N-terminus. Moreover, testing of all 20 amino acids in the position after the initiator methionine revealed strong differences in protein stability and indicated an important role of the penultimate N-terminal amino acid residue in determining the protein half life. We propose that the stability of plastid proteins is largely determined by three factors: (i) the action of methionine aminopeptidase (the enzyme that removes the initiator methionine and exposes the penultimate N-terminal amino acid residue), (ii) an N-end rule-like protein degradation pathway, and (iii) additional sequence determinants in the N-terminal region.
© 2010 The Authors. Journal compilation © 2010 Blackwell Publishing Ltd.
Figures



References
-
- Adam Z. Protein stability and degradation in chloroplasts. Plant Mol. Biol. 1996;32:773–783. - PubMed
-
- Adam Z. Chloroplast proteases: Possible regulators of gene expression. Biochimie. 2000;82:647–654. - PubMed
-
- Adam Z. Protein stability and degradation in plastids. Top. Curr. Genet. 2007;19:315–338.
-
- Adam Z, Rudella A, van Wijk KJ. Recent advances in the study of Clp, FtsH and other proteases located in chloroplasts. Curr. Opin. Plant Biol. 2006;9:234–240. - PubMed
-
- Barber J, Nield J, Morris EP, Zheleva D, Hankamer B. The structure, function and dynamics of photosystem two. Physiol. Plant. 1997;100:817–827.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

