Differences in quality between privately and publicly banked umbilical cord blood units: a pilot study of autologous cord blood infusion in children with acquired neurologic disorders
- PMID: 20546200
- PMCID: PMC3816574
- DOI: 10.1111/j.1537-2995.2010.02720.x
Differences in quality between privately and publicly banked umbilical cord blood units: a pilot study of autologous cord blood infusion in children with acquired neurologic disorders
Abstract
Background: A pilot study was conducted to determine the safety and feasibility of intravenous administration of autologous umbilical cord blood (CB) in young children with acquired neurologic disorders. Most CB units (CBUs) were electively stored in private CB banks. Unlike public banks, which utilize specific criteria and thresholds for banking, private banks generally store all collected CBUs.
Study design and methods: CBUs of eligible patients containing more than 1 × 10⁷ cells/kg were shipped to Duke from the banks of origin after confirming identity by HLA typing. On the day of infusion, CBUs were thawed and washed in dextran-albumin and infused intravenously. Patients were medicated with acetaminophen, diphenhydramine, and methylprednisolone before transfusion. Data regarding patients, infusions, and CBUs were collected retrospectively. Characteristics of CBUs were compared to existing data from CBUs publicly banked at the Carolinas Cord Blood Bank.
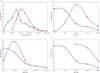
Results: From March 2004 to December 2009, 184 children received 198 CB infusions. Three patients had infusion reactions, all responsive to medical therapy and stopping the infusion. Median precryopreservation volume (60 mL vs. 89 mL, p < 0.0001), total nucleated cell count (4.7 × 10⁸ vs. 10.8 × 10⁸, p < 0.0001), and CD34 count (1.8 × 10⁶ vs. 3.0 × 10⁶, p < 0.0001) were significantly lower than publicly stored CBUs. Postthaw sterility cultures were positive in 7.6% of infused CBUs.
Conclusion: IV infusion of autologous CB is safe and feasible in young children with neurologic injuries. Quality parameters of privately banked CBUs are inferior to those stored in public banks. If efficacy of autologous CB is established clinically, the quality of autologous units should be held to the same standards as those stored in public banks.
© 2010 American Association of Blood Banks.
Conflict of interest statement
The authors declare that they have no conflicts of interest relevant to the manuscript submitted to
Figures
Comment in
-
Between the trash can and the freezer: donor education and the fate of cord blood.Transfusion. 2011 Feb;51(2):234-6. doi: 10.1111/j.1537-2995.2010.03018.x. Transfusion. 2011. PMID: 21309775 No abstract available.
References
-
- Lu D, Sanberg PR, Mahmood A, Li Y, Wang L, Sanchez-Ramos J, Chopp M. Intravenous administration of human umbilical cord blood reduces neurological deficit in the rat after traumatic brain injury. Cell Transplant. 2002;11:275–281. - PubMed
-
- Nishio Y, Koda M, Kamada T, Someya Y, Yoshinaga K, Okada S, Harada H, Okawa A, Moriya H, Yamazaki M. The use of hemopoietic stem cells derived from human umbilical cord blood to promote restoration of spinal cord tissue and recovery of hindlimb function in adult rats. J Neurosurg Spine. 2006;5:424–433. - PubMed
-
- Nan Z, Grande A, Sanberg CD, Sanberg PR, Low WC. Infusion of human umbilical cord blood ameliorates neurologic deficits in rats with hemorrhagic brain injury. Ann N Y Acad Sci. 2005;1049:84–96. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

