Expansion of mouse sertoli cells on microcarriers
- PMID: 20546245
- PMCID: PMC6496594
- DOI: 10.1111/j.1365-2184.2010.00677.x
Expansion of mouse sertoli cells on microcarriers
Abstract
Background: Sertoli cells (SCs) have been described as the 'nurse cells' of the testis whose primary function is to provide essential growth factors and create an appropriate environment for development of other cells [for example, germinal and nerve stem cells (NSCs), used here]. However, the greatest challenge at present is that it is difficult to obtain sufficient SCs of normal physiological function for cell transplantation and biological medicine, largely due to traditional static culture parameter difficult to be monitored and scaled up.
Objective: Operational stirred culture conditions for in vitro expansion and differentiation of SCs need to be optimized for large-scale culture.
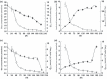
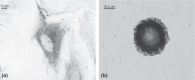
Materials and methods: In this study, the culturing process for primary SC expansion and maintaining lack of differentiation was optimized for the first time, by using microcarrier bead technology in spinner flask culture. Effects of various feeding/refreshing regimes, stirring speeds, seed inoculum levels of SCs, and concentrations of microcarrier used for expansion of mouse SCs were also explored. In addition, pH, osmotic pressure and metabolic variables including consumption rates of glucose, glutamine, amino acids, and formation rates of lactic acid and ammonia, were investigated in culture.
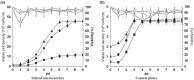
Results: After 6 days, maximal cell densities achieved were 4.6 x 10(6) cells/ml for Cytodex-1 in DMEM/FBS compared to 4.8 x 10(5) cells/ml in static culture. Improved expansion was achieved using an inoculum of 1 x 10(5) cells/ml and microcarrier concentration of 3 mg/ml at stirring speed of 30 rpm. RESULTS indicated that medium replacement (50% changed everyday) resulted in supply of nutrients and removal of waste products inhibiting cell growth, that lead to maintenance of cultures in steady state for several days. These conditions favoured preservation of SCs in the undifferentiated state and significantly increased their physiological activity and trophic function, which were assessed by co-culturing with NSCs and immunostaining.
Conclusion: Data obtained in this study demonstrate the vast potential of this stirred culture system for efficient, reproducible and cost-effective expansion of SCs in vitro. The system has advantages over static culture, which has major obstacles such as lower cell density, is time-consuming and susceptible to contamination.
Figures





References
-
- Fawcett DW (1975) In: Hamilton DW, Grup RD, eds. Handbook of Physiology: Male Reproductive System, Vol. 5, pp. 21–56, Washington, DC: American Physiological Society.
-
- Skinner MK 1993. Secretion of growth factors and other regulatory factors In: Russell LA, Griswold MD, eds. The Sertoli Cell, pp. 237–248. Clearwater: Cache River Press.
-
- Loveland KL, Zlatic K, Stein‐Oakley A, Risbridger G, DeKretser DM (1995) Platelet‐derived growth factor ligand and receptor subunit mRNA in the Sertoli and Leydig cells of the rat testis. Mol. Cell. Endocrinol. 108, 155–159. - PubMed
-
- Selawry HP, Cameron DF (1993) Sertoli cell‐enriched fractions in successful islet cell transplantation. Cell Transplant. 2, 123–129. - PubMed
-
- Widenfalk J, Nosrat C, Tomac A, Westphal H, Hoffer B, Olson L (1997) Neurturin and glial cell line‐derived neurotrophic factor receptor‐beta (GDNFR‐beta), novel proteins related to GDNF and GDNFR‐alpha with specific cellular patterns of expression suggesting roles in the developing and adult nervous system and in peripheral organs. J. Neurosci. 17, 8506–8519. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

