Expression profiling of Crambe abyssinica under arsenate stress identifies genes and gene networks involved in arsenic metabolism and detoxification
- PMID: 20546591
- PMCID: PMC3095275
- DOI: 10.1186/1471-2229-10-108
Expression profiling of Crambe abyssinica under arsenate stress identifies genes and gene networks involved in arsenic metabolism and detoxification
Abstract
Background: Arsenic contamination is widespread throughout the world and this toxic metalloid is known to cause cancers of organs such as liver, kidney, skin, and lung in human. In spite of a recent surge in arsenic related studies, we are still far from a comprehensive understanding of arsenic uptake, detoxification, and sequestration in plants. Crambe abyssinica, commonly known as 'abyssinian mustard', is a non-food, high biomass oil seed crop that is naturally tolerant to heavy metals. Moreover, it accumulates significantly higher levels of arsenic as compared to other species of the Brassicaceae family. Thus, C. abyssinica has great potential to be utilized as an ideal inedible crop for phytoremediation of heavy metals and metalloids. However, the mechanism of arsenic metabolism in higher plants, including C. abyssinica, remains elusive.
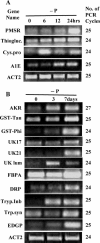
Results: To identify the differentially expressed transcripts and the pathways involved in arsenic metabolism and detoxification, C. abyssinica plants were subjected to arsenate stress and a PCR-Select Suppression Subtraction Hybridization (SSH) approach was employed. A total of 105 differentially expressed subtracted cDNAs were sequenced which were found to represent 38 genes. Those genes encode proteins functioning as antioxidants, metal transporters, reductases, enzymes involved in the protein degradation pathway, and several novel uncharacterized proteins. The transcripts corresponding to the subtracted cDNAs showed strong upregulation by arsenate stress as confirmed by the semi-quantitative RT-PCR.
Conclusions: Our study revealed novel insights into the plant defense mechanisms and the regulation of genes and gene networks in response to arsenate toxicity. The differential expression of transcripts encoding glutathione-S-transferases, antioxidants, sulfur metabolism, heat-shock proteins, metal transporters, and enzymes in the ubiquitination pathway of protein degradation as well as several unknown novel proteins serve as molecular evidence for the physiological responses to arsenate stress in plants. Additionally, many of these cDNA clones showing strong upregulation due to arsenate stress could be used as valuable markers. Further characterization of these differentially expressed genes would be useful to develop novel strategies for efficient phytoremediation as well as for engineering arsenic tolerant crops with reduced arsenic translocation to the edible parts of plants.
Figures


Similar articles
-
Identifying genes and gene networks involved in chromium metabolism and detoxification in Crambe abyssinica.Environ Pollut. 2011 Oct;159(10):3123-8. doi: 10.1016/j.envpol.2011.06.027. Epub 2011 Jul 23. Environ Pollut. 2011. PMID: 21784565
-
Do heavy metals and metalloids influence the detoxification of organic xenobiotics in plants?Environ Sci Pollut Res Int. 2009 Nov;16(7):795-804. doi: 10.1007/s11356-009-0168-7. Epub 2009 May 22. Environ Sci Pollut Res Int. 2009. PMID: 19462193
-
Identification of differentially-expressed genes potentially implicated in drought response in pitaya (Hylocereus undatus) by suppression subtractive hybridization and cDNA microarray analysis.Gene. 2014 Jan 1;533(1):322-31. doi: 10.1016/j.gene.2013.08.098. Epub 2013 Sep 27. Gene. 2014. PMID: 24076355
-
Implications of metal accumulation mechanisms to phytoremediation.Environ Sci Pollut Res Int. 2009 Mar;16(2):162-75. doi: 10.1007/s11356-008-0079-z. Epub 2008 Dec 6. Environ Sci Pollut Res Int. 2009. PMID: 19067014 Review.
-
Two facets of world arsenic problem solution: crop poisoning restriction and enforcement of phytoremediation.Planta. 2018 Jul;248(1):19-35. doi: 10.1007/s00425-018-2906-x. Epub 2018 May 7. Planta. 2018. PMID: 29736625 Review.
Cited by
-
Transcriptome profiling of genes and pathways associated with arsenic toxicity and tolerance in Arabidopsis.BMC Plant Biol. 2014 Apr 16;14:94. doi: 10.1186/1471-2229-14-94. BMC Plant Biol. 2014. PMID: 24734953 Free PMC article.
-
Target proteins reprogrammed by As and As + Si treatments in Solanum lycopersicum L. fruit.BMC Plant Biol. 2017 Nov 21;17(1):210. doi: 10.1186/s12870-017-1168-2. BMC Plant Biol. 2017. PMID: 29157202 Free PMC article.
-
Sulfur Mediated Alleviation of Mn Toxicity in Polish Wheat Relates to Regulating Mn Allocation and Improving Antioxidant System.Front Plant Sci. 2016 Sep 15;7:1382. doi: 10.3389/fpls.2016.01382. eCollection 2016. Front Plant Sci. 2016. PMID: 27695467 Free PMC article.
-
A γ-glutamyl cyclotransferase protects Arabidopsis plants from heavy metal toxicity by recycling glutamate to maintain glutathione homeostasis.Plant Cell. 2013 Nov;25(11):4580-95. doi: 10.1105/tpc.113.111815. Epub 2013 Nov 8. Plant Cell. 2013. PMID: 24214398 Free PMC article.
-
Proteomics analysis identified a DRT protein involved in arsenic resistance in Populus.Plant Cell Rep. 2017 Dec;36(12):1855-1869. doi: 10.1007/s00299-017-2199-8. Epub 2017 Aug 16. Plant Cell Rep. 2017. PMID: 28815368
References
-
- World Health Organization (WHO) Arsenic in drinking water, Fact sheet No. 210. http://www.who.int/int-fs/en/fact210.html
-
- Baker RS, Arle HF, Miller JH, Holstun JT. Effects of organic arsenical herbicides on cotton response and chemical residues. Weed Sci. 1969;17:37–40.
-
- US Department of Agriculture. Wood Preservatives. The Pesticide Review. Washington DC. 1974.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

