Decorin transfection suppresses profibrogenic genes and myofibroblast formation in human corneal fibroblasts
- PMID: 20546727
- PMCID: PMC2929567
- DOI: 10.1016/j.exer.2010.05.013
Decorin transfection suppresses profibrogenic genes and myofibroblast formation in human corneal fibroblasts
Abstract
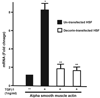
Decorin, a small leucine-rich proteoglycan, is a natural inhibitor of transforming growth factor beta (TGFbeta). Myofibroblast and haze formation in the cornea have been attributed to TGFbeta hyperactivity released from corneal epithelium following injury to eye. This study tested the hypothesis that decorin-gene transfer inhibits TGFbeta-driven myofibroblast and haze formation in the cornea. Human corneal fibroblast (HSF) cultures generated from donor human corneas were used. Decorin cDNA was cloned into mammalian expression vector. Restriction enzyme analysis and DNA sequencing confirmed the nucleotide sequence of generated vector construct. The decorin gene cloned into mammalian expression vector was introduced into HSF with lipofectamine transfection kit. Expression of decorin in selected clones was characterized with RT-PCR, immunocytochemistry and western blotting. Phage contrast microscopy and trypan blue exclusion assay evaluated the effects of decorin-gene transfer on HSF phenotype and viability, respectively. Real-time PCR, western blot and immunocytochemistry were used to analyze inhibitory effects of decorin-gene transfer on TGFbeta-induced myofibroblast formation by measuring differential expression of alpha smooth muscle actin (SMA), a myofibroblast marker, mRNA and protein expression. Analysis of variance (ANOVA) and the Bonferroni-Dunn adjustment for repeated measures were used for statistical analysis. Our data indicate that decorin-gene transfer into HSF do not alter cellular phenotype or viability. Decorin over-expressing HSF clones grown in the presence of TGFbeta1 under serum-free conditions showed a statistically significant 80-83% decrease in SMA expression (p value < 0.01) compared to naked-vector transfected clones or un-transfected HSF controls. Decorin-transfected, naked-vector transfected and un-transfected HSF grown in the absence of TGFbeta1 showed no or extremely low expression of SMA. Furthermore, decorin over-expression did not affect HSF phenotype and decreased TGFbeta-induced RNA levels of profibrogenic genes such as fibronectin, collagen type I, III, and IV that play important role in stromal matrix modulation and corneal wound healing. The results of study suggest that decorin-gene transfer effectively prevents TGFbeta-driven transformation of keratocyte and corneal fibroblast to myofibroblasts. We postulate that decorin-gene therapy can be used to treat corneal haze in vivo.
Published by Elsevier Ltd.
Figures





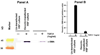
References
-
- Bainbridge JW, Smith AJ, Barker SS, Robbie S, Henderson R, Balaggan K, Viswanathan A, Holder GE, Stockman A, Tyler N, Petersen-Jones S, Bhattacharya SS, Thrasher AJ, Fitzke FW, Carter BJ, Rubin GS, Moore AT, Ali RR. Effect of gene therapy on visual function in Leber's congenital amaurosis. N. Engl. J. Med. 2008;358:2231–2239. - PubMed
-
- Behrens A, Gordon EM, Li L, Liu PX, Chen Z, Peng H, La Bree L, Anderson WF, Hall FL, McDonnell PJ. Retroviral gene therapy vectors for prevention of excimer laser-induced corneal haze. Invest Ophthalmol Vis Sci. 2002;43:968–977. - PubMed
-
- Border WA, Noble NA, Yamamoto T, Harper JR, Yamaguchi Y, Pierschbacher MD, Ruoslahti E. Natural inhibitor of transforming growth factor-beta protects against scarring in experimental kidney disease. Nature. 1992;360:361–364. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

