CD39/ENTPD1 expression by CD4+Foxp3+ regulatory T cells promotes hepatic metastatic tumor growth in mice
- PMID: 20546740
- PMCID: PMC2930043
- DOI: 10.1053/j.gastro.2010.05.007
CD39/ENTPD1 expression by CD4+Foxp3+ regulatory T cells promotes hepatic metastatic tumor growth in mice
Abstract
Background & aims: Adenosine mediates immune suppression and is generated by the ectonucleotidases CD39 (ENTPD1) and CD73 that are expressed on vascular endothelial cells and regulatory T cells (Tregs). Although tumor-infiltrating immune cells include Foxp3(+) Tregs, it is not clear whether local adenosine generation by Tregs promotes tumor growth in a CD39-dependent manner. In this study, we have examined the effect of CD39 expression by Tregs on effector immune cell responses to hepatic metastases in vivo.
Methods: A model of hepatic metastatic cancer was developed with portal vein infusion of luciferase-expressing melanoma B16/F10 cells and MCA38 colon cancer cells in wild-type (wt) and mutant mice null for Cd39. Chimeric mice were generated by bone marrow transplantation (BMT) using Cd39 null or wt C57BL6 donors and irradiated recipient mice.
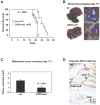
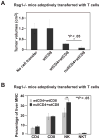
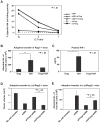
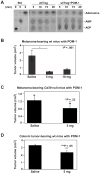
Results: We demonstrate that hepatic growth of melanoma metastatic tumors was strongly inhibited in mice with Cd39 null vasculature or in wt mice with circulating Cd39 null bone marrow-derived cells. We show functional CD39 expression on CD4(+)Foxp3(+) Tregs suppressed antitumor immunity mediated by natural killer (NK) cells in vitro and in vivo. Finally, inhibition of CD39 activity by polyoxometalate-1, a pharmacologic inhibitor of nucleoside triphosphate diphosphohydrolase activity, significantly inhibited tumor growth (P < .001).
Conclusions: CD39 expression on Tregs inhibits NK activity and is permissive for metastatic growth. Pharmacologic or targeted inhibition of CD39 enzymatic activity may find utility as an adjunct therapy for secondary hepatic malignancies.
Copyright © 2010 AGA Institute. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures



References
-
- Ogawa T, Takayama K, Takakura N, Kitano S, Ueno H. Anti-tumor angiogenesis therapy using soluble receptors: enhanced inhibition of tumor growth when soluble fibroblast growth factor receptor-1 is used with soluble vascular endothelial growth factor receptor. Cancer Gene Ther. 2002;9:633–40. - PubMed
-
- Chari RS, Helton WS, Marsh RD. Chemotherapy and regional therapy of hepatic colorectal metastases: expert consensus statement by Bartlett et al. Ann Surg Oncol. 2006;13:1293–5. - PubMed
-
- Hoskin DW, Reynolds T, Blay J. Adenosine as a possible inhibitor of killer T-cell activation in the microenvironment of solid tumours. Int J Cancer. 1994;59:854–5. - PubMed
-
- Burnstock G. Purinergic signaling and vascular cell proliferation and death. Arterioscler Thromb Vasc Biol. 2002;22:364–73. - PubMed
-
- Spychala J. Tumor-promoting functions of adenosine. Pharmacol Ther. 2000;87:161–73. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

