Purified Plasmodium falciparum multi-drug resistance protein (PfMDR 1) binds a high affinity chloroquine analogue
- PMID: 20546803
- PMCID: PMC2906614
- DOI: 10.1016/j.molbiopara.2010.05.012
Purified Plasmodium falciparum multi-drug resistance protein (PfMDR 1) binds a high affinity chloroquine analogue
Abstract
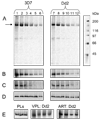
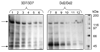
We utilize the recent successful overexpression of recombinant Plasmodium falciparum multi-drug resistance transporter, purification and reconstitution of the protein, and a novel high affinity chloroquine analogue to probe hypothesized interaction between the transporter and quinoline drugs. Results suggest that PfMDR1 binding sites for chloroquine, mefloquine, and quinine overlap, that P. falciparum chloroquine resistance transporter has intrinsically higher affinity for chloroquine relative to P. falciparum multi-drug resistance transporter, and that there is an isoform specific competition between the two transporters for binding of quinoline antimalarial drugs.
Copyright 2010 Elsevier B.V. All rights reserved.
Figures
Similar articles
-
A 2-amino quinoline, 5-(3-(2-(7-chloroquinolin-2-yl)ethenyl)phenyl)-8-dimethylcarbamyl-4,6-dithiaoctanoic acid, interacts with PfMDR1 and inhibits its drug transport in Plasmodium falciparum.Mol Biochem Parasitol. 2014 Jun;195(1):34-42. doi: 10.1016/j.molbiopara.2014.05.006. Epub 2014 Jun 8. Mol Biochem Parasitol. 2014. PMID: 24914817
-
Multiple drugs compete for transport via the Plasmodium falciparum chloroquine resistance transporter at distinct but interdependent sites.J Biol Chem. 2014 Dec 26;289(52):36336-51. doi: 10.1074/jbc.M114.614206. Epub 2014 Nov 6. J Biol Chem. 2014. PMID: 25378409 Free PMC article.
-
Chemical Proteomics and Super-resolution Imaging Reveal That Chloroquine Interacts with Plasmodium falciparum Multidrug Resistance-Associated Protein and Lipids.ACS Chem Biol. 2018 Oct 19;13(10):2939-2948. doi: 10.1021/acschembio.8b00583. Epub 2018 Sep 24. ACS Chem Biol. 2018. PMID: 30208272
-
Transporters involved in resistance to antimalarial drugs.Trends Pharmacol Sci. 2006 Nov;27(11):594-601. doi: 10.1016/j.tips.2006.09.005. Epub 2006 Sep 25. Trends Pharmacol Sci. 2006. PMID: 16996622 Free PMC article. Review.
-
Quinoline antimalarials: mechanisms of action and resistance.Int J Parasitol. 1997 Feb;27(2):231-40. doi: 10.1016/s0020-7519(96)00152-x. Int J Parasitol. 1997. PMID: 9088993 Review.
Cited by
-
Recombinant vacuolar iron transporter family homologue PfVIT from human malaria-causing Plasmodium falciparum is a Fe2+/H+exchanger.Sci Rep. 2017 Feb 15;7:42850. doi: 10.1038/srep42850. Sci Rep. 2017. PMID: 28198449 Free PMC article.
-
Plasmodium falciparum resistance to cytocidal versus cytostatic effects of chloroquine.Mol Biochem Parasitol. 2011 Jul-Aug;178(1-2):1-6. doi: 10.1016/j.molbiopara.2011.03.003. Epub 2011 Apr 4. Mol Biochem Parasitol. 2011. PMID: 21470564 Free PMC article.
-
Chloroquine susceptibility and reversibility in a Plasmodium falciparum genetic cross.Mol Microbiol. 2010 Nov;78(3):770-87. doi: 10.1111/j.1365-2958.2010.07366.x. Epub 2010 Sep 29. Mol Microbiol. 2010. PMID: 20807203 Free PMC article.
-
PfCRT-mediated drug transport in malarial parasites.Biochemistry. 2011 Jan 18;50(2):163-71. doi: 10.1021/bi101638n. Epub 2010 Dec 22. Biochemistry. 2011. PMID: 21142008 Free PMC article. Review.
-
Mechanistic basis for multidrug resistance and collateral drug sensitivity conferred to the malaria parasite by polymorphisms in PfMDR1 and PfCRT.PLoS Biol. 2022 May 4;20(5):e3001616. doi: 10.1371/journal.pbio.3001616. eCollection 2022 May. PLoS Biol. 2022. PMID: 35507548 Free PMC article.
References
-
- Wellems TE, Panton LJ, Gluzman IY, do Rosario VE, Gwadz RW, Walker-Jonah A, Krogstad DJ. Chloroquine resistance not linked to mdr-like genes in a Plasmodium falciparum cross. Nature. 1990;345:253–255. - PubMed
-
- Wilson CM, Serrano AE, Wasley A, Bogenschutz MP, Shankar AH, Wirth DF. Amplification of a gene related to mammalian mdr genes in drug-resistant Plasmodium falciparum. Science. 1989;244(4909):1184–1186. 9. - PubMed
-
- Fidock DA, Nomura T, Talley AK, Cooper RA, Dzekunov SM, Ferdig MT, Ursos LM, Sidhu AB, Naude B, Deitsch KW, Su XZ, Wootton JC, Roepe PD, Wellems TE. Mutations in the P. falciparum digestive vacuole transmembrane protein PfCRT and evidence for their role in chloroquine resistance. Mol Cell. 2000;6(4):861–871. - PMC - PubMed
-
- Reed MB, Saliba KJ, Caruana SR, Kirk K, Cowman AF. Pgh1 modulates sensitivity and resistance to multiple antimalarials in Plasmodium falciparum. Nature. 2000;403(6772):906–909. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

