Drug delivery to solid tumors by elastin-like polypeptides
- PMID: 20546809
- PMCID: PMC2940962
- DOI: 10.1016/j.addr.2010.05.004
Drug delivery to solid tumors by elastin-like polypeptides
Abstract
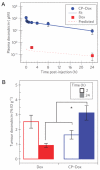
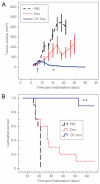
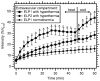
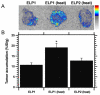
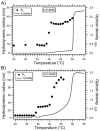
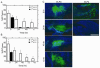
Thermally responsive elastin-like polypeptides (ELPs) are a promising class of recombinant biopolymers for the delivery of drugs and imaging agents to solid tumors via systemic or local administration. This article reviews four applications of ELPs to drug delivery, with each delivery mechanism designed to best exploit the relationship between the characteristic transition temperature (T(t)) of the ELP and body temperature (T(b)). First, when T(t)≫T(b), small hydrophobic drugs can be conjugated to the C-terminus of the ELP to impart the amphiphilicity needed to mediate the self-assembly of nanoparticles. These systemically delivered ELP-drug nanoparticles preferentially localize to the tumor site via the EPR effect, resulting in reduced toxicity and enhanced treatment efficacy. The remaining three approaches take direct advantage of the thermal responsiveness of ELPs. In the second strategy, where T(b)<T(t)<42°C, an ELP-drug conjugate can be injected in conjunction with external application of mild hyperthermia to the tumor to induce ELP coacervation and an increase in concentration within the tumor vasculature. The third approach utilizes hydrophilic-hydrophobic ELP block copolymers that have been designed to assemble into nanoparticles in response to hyperthermai due to the independent thermal transition of the hydrophobic block, thus resulting in multivalent ligand display of a ligand for spatially enhanced vascular targeting. In the final strategy, ELPs with T(t)<T(b) are conjugated with radiotherapeutics, injtect intioa tumor where they undergo coacervation to form an injectable drug depot for intratumoral delivery. These injectable coacervate ELP-radionuclide depots display a long residence in the tumor and result in inhibition of tumor growth.
Copyright © 2010 Elsevier B.V. All rights reserved.
Figures





References
-
- Duncan R. Polymer conjugates as anticancer nanomedicines. Nature Reviews Cancer. 2006;6(9):688–701. - PubMed
-
- Vasey PA, Kaye SB, Morrison R, Twelves C, Wilson P, Duncan R, Thomson AH, Murray LS, Hilditch TE, Murray T, Burtles S, Fraier D, Frigerio E, Cassidy J, I.I.I.C. Canc Res Campaign Phase Phase i clinical and pharmacokinetic study of pk1 n-(2-hydroxypropyl)methacrylamide copolymer doxorubicin : First member of a new class of chemotherapeutic agents - drug-polymer conjugates. Clinical Cancer Research. 1999;5(1):83–94. - PubMed
-
- Chuang VTG, Kragh-Hansen U, Otagiri M. Pharmaceutical strategies utilizing recombinant human serum albumin. Pharmaceutical Research. 2002;19(5):569–577. - PubMed
-
- Vandamme TF, Lenourry A, Charrueau C, Chaumeil J. The use of polysaccharides to target drugs to the colon. Carbohydrate Polymers. 2002;48(3):219–231.
-
- Allen TM. Liposomes - opportunities in drug delivery. Drugs. 1997;54:8–14. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

