Assessing spinal axon regeneration and sprouting in Nogo-, MAG-, and OMgp-deficient mice
- PMID: 20547125
- PMCID: PMC2896331
- DOI: 10.1016/j.neuron.2010.05.002
Assessing spinal axon regeneration and sprouting in Nogo-, MAG-, and OMgp-deficient mice
Abstract
A central hypothesis for the limited capacity for adult central nervous system (CNS) axons to regenerate is the presence of myelin-derived axon growth inhibitors, the role of which, however, remains poorly understood. We have conducted a comprehensive genetic analysis of the three major myelin inhibitors, Nogo, MAG, and OMgp, in injury-induced axonal growth, including compensatory sprouting of uninjured axons and regeneration of injured axons. While deleting any one inhibitor in mice enhanced sprouting of corticospinal or raphespinal serotonergic axons, there was neither associated behavioral improvement nor a synergistic effect of deleting all three inhibitors. Furthermore, triple-mutant mice failed to exhibit enhanced regeneration of either axonal tract after spinal cord injury. Our data indicate that while Nogo, MAG, and OMgp may modulate axon sprouting, they do not play a central role in CNS axon regeneration failure.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures
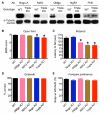
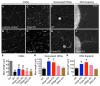
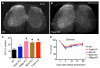
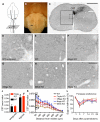

Comment in
-
Much Ado about Nogo.Neuron. 2010 Jun 10;66(5):619-21. doi: 10.1016/j.neuron.2010.05.028. Neuron. 2010. PMID: 20547119
References
-
- Atwal JK, Pinkston-Gosse J, Syken J, Stawicki S, Wu Y, Shatz C, Tessier-Lavigne M. PirB is a functional receptor for myelin inhibitors of axonal regeneration. Science. 2008;322:967–970. - PubMed
-
- Bartsch U, Bandtlow CE, Schnell L, Bartsch S, Spillmann AA, Rubin BP, Hillenbrand R, Montag D, Schwab ME, Schachner M. Lack of evidence that myelin-associated glycoprotein is a major inhibitor of axonal regeneration in the CNS. Neuron. 1995;15:1375–1381. - PubMed
-
- Basso DM, Fisher LC, Anderson AJ, Jakeman LB, McTigue DM, Popovich PG. Basso Mouse Scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J Neurotrauma. 2006;23:635–659. - PubMed
-
- Bradbury EJ, McMahon SB. Spinal cord repair strategies: why do they work? Nat Rev Neurosci. 2006;7:644–653. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

