The Dnmt3a PWWP domain reads histone 3 lysine 36 trimethylation and guides DNA methylation
- PMID: 20547484
- PMCID: PMC2924014
- DOI: 10.1074/jbc.M109.089433
The Dnmt3a PWWP domain reads histone 3 lysine 36 trimethylation and guides DNA methylation
Abstract
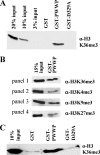
The Dnmt3a DNA methyltransferase contains in its N-terminal part a PWWP domain that is involved in chromatin targeting. Here, we have investigated the interaction of the PWWP domain with modified histone tails using peptide arrays and show that it specifically recognizes the histone 3 lysine 36 trimethylation mark. H3K36me3 is known to be a repressive modification correlated with DNA methylation in mammals and heterochromatin in Schizosaccharomyces pombe. These results were confirmed by equilibrium peptide binding studies and pulldown experiments with native histones and purified native nucleosomes. The PWWP-H3K36me3 interaction is important for the subnuclear localization of enhanced yellow fluorescent protein-fused Dnmt3a. Furthermore, the PWWP-H3K36me3 interaction increases the activity of Dnmt3a for methylation of nucleosomal DNA as observed using native nucleosomes isolated from human cells after demethylation of the DNA with 5-aza-2'-deoxycytidine as substrate for methylation with Dnmt3a. These data suggest that the interaction of the PWWP domain with H3K36me3 is involved in targeting of Dnmt3a to chromatin carrying that mark, a model that is in agreement with several studies on the genome-wide distribution of DNA methylation and H3K36me3.
Figures


References
-
- Goll M. G., Bestor T. H. (2005) Annu. Rev. Biochem. 74, 481–514 - PubMed
-
- Klose R. J., Bird A. P. (2006) Trends Biochem. Sci. 31, 89–97 - PubMed
-
- Reik W. (2007) Nature 447, 425–432 - PubMed
-
- Okano M., Bell D. W., Haber D. A., Li E. (1999) Cell 99, 247–257 - PubMed
-
- Bourc'his D., Xu G. L., Lin C. S., Bollman B., Bestor T. H. (2001) Science 294, 2536–2539 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

