Substrate control of internal electron transfer in bacterial nitric-oxide reductase
- PMID: 20547487
- PMCID: PMC2919117
- DOI: 10.1074/jbc.M110.123984
Substrate control of internal electron transfer in bacterial nitric-oxide reductase
Abstract
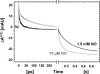
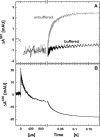
Nitric -oxide reductase (NOR) from Paracoccus denitrificans catalyzes the reduction of nitric oxide (NO) to nitrous oxide (N(2)O) (2NO + 2H(+) + 2e(-) -->N(2)O + H(2)O) by a poorly understood mechanism. NOR contains two low spin hemes c and b, one high spin heme b(3), and a non-heme iron Fe(B). Here, we have studied the reaction between fully reduced NOR and NO using the "flow-flash" technique. Fully (four-electron) reduced NOR is capable of two turnovers with NO. Initial binding of NO to reduced heme b(3) occurs with a time constant of approximately 1 micros at 1.5 mM NO, in agreement with earlier studies. This reaction is [NO]-dependent, ruling out an obligatory binding of NO to Fe(B) before ligation to heme b(3). Oxidation of hemes b and c occurs in a biphasic reaction with rate constants of 50 s(-1) and 3 s(-1) at 1.5 mM NO and pH 7.5. Interestingly, this oxidation is accelerated as [NO] is lowered; the rate constants are 120 s(-1) and 12 s(-1) at 75 microM NO. Protons are taken up from solution concomitantly with oxidation of the low spin hemes, leading to an acceleration at low pH. This effect is, however, counteracted by a larger degree of substrate inhibition at low pH. Our data thus show that substrate inhibition in NOR, previously observed during multiple turnovers, already occurs during a single oxidative cycle. Thus, NO must bind to its inhibitory site before electrons redistribute to the active site. The further implications of our data for the mechanism of NO reduction by NOR are discussed.
Figures


Similar articles
-
Electron/proton coupling in bacterial nitric oxide reductase during reduction of oxygen.Biochemistry. 2005 Aug 9;44(31):10711-9. doi: 10.1021/bi050524h. Biochemistry. 2005. PMID: 16060680
-
Proton and electron pathways in the bacterial nitric oxide reductase.Biochemistry. 2002 Feb 19;41(7):2331-40. doi: 10.1021/bi0121050. Biochemistry. 2002. PMID: 11841226
-
Electron transfer to the active site of the bacterial nitric oxide reductase is controlled by ligand binding to heme b₃.Biochim Biophys Acta. 2011 Apr;1807(4):451-7. doi: 10.1016/j.bbabio.2011.01.009. Epub 2011 Feb 4. Biochim Biophys Acta. 2011. PMID: 21296048
-
Proton transfer in bacterial nitric oxide reductase.Biochem Soc Trans. 2006 Feb;34(Pt 1):188-90. doi: 10.1042/BST0340188. Biochem Soc Trans. 2006. PMID: 16417518 Review.
-
The bacterial respiratory nitric oxide reductase.Biochem Soc Trans. 2009 Apr;37(Pt 2):392-9. doi: 10.1042/BST0370392. Biochem Soc Trans. 2009. PMID: 19290869 Review.
Cited by
-
Investigating the Proton Donor in the NO Reductase from Paracoccus denitrificans.PLoS One. 2016 Mar 31;11(3):e0152745. doi: 10.1371/journal.pone.0152745. eCollection 2016. PLoS One. 2016. PMID: 27030968 Free PMC article.
-
Characterization of the quinol-dependent nitric oxide reductase from the pathogen Neisseria meningitidis, an electrogenic enzyme.Sci Rep. 2018 Feb 26;8(1):3637. doi: 10.1038/s41598-018-21804-0. Sci Rep. 2018. PMID: 29483528 Free PMC article.
-
The nitric-oxide reductase from Paracoccus denitrificans uses a single specific proton pathway.J Biol Chem. 2013 Oct 18;288(42):30626-30635. doi: 10.1074/jbc.M113.497347. Epub 2013 Sep 6. J Biol Chem. 2013. PMID: 24014024 Free PMC article.
-
Recent advances in biosynthetic modeling of nitric oxide reductases and insights gained from nuclear resonance vibrational and other spectroscopic studies.Inorg Chem. 2015 Oct 5;54(19):9317-29. doi: 10.1021/acs.inorgchem.5b01105. Epub 2015 Aug 14. Inorg Chem. 2015. PMID: 26274098 Free PMC article.
-
Assay development and inhibition of the Mt-DprE2 essential reductase from Mycobacterium tuberculosis.Microbiology (Reading). 2023 Jan;169(1):001288. doi: 10.1099/mic.0.001288. Microbiology (Reading). 2023. PMID: 36748627 Free PMC article.
References
-
- Zumft W. G. (2005) J. Inorg. Biochem. 99, 194–215 - PubMed
-
- Watmough N. J., Field S. J., Hughes R. J., Richardson D. J. (2009) Biochem. Soc. Trans. 37, 392–399 - PubMed
-
- Saraste M., Castresana J. (1994) FEBS Lett. 341, 1–4 - PubMed
-
- van der Oost J., de Boer A. P., de Gier J. W., Zumft W. G., Stouthamer A. H., van Spanning R. J. (1994) FEMS Microbiol. Lett. 121, 1–9 - PubMed
-
- Hendriks J., Warne A., Gohlke U., Haltia T., Ludovici C., Lübben M., Saraste M. (1998) Biochemistry 37, 13102–13109 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

