Generalizing evidence from randomized clinical trials to target populations: The ACTG 320 trial
- PMID: 20547574
- PMCID: PMC2915476
- DOI: 10.1093/aje/kwq084
Generalizing evidence from randomized clinical trials to target populations: The ACTG 320 trial
Abstract
Properly planned and conducted randomized clinical trials remain susceptible to a lack of external validity. The authors illustrate a model-based method to standardize observed trial results to a specified target population using a seminal human immunodeficiency virus (HIV) treatment trial, and they provide Monte Carlo simulation evidence supporting the method. The example trial enrolled 1,156 HIV-infected adult men and women in the United States in 1996, randomly assigned 577 to a highly active antiretroviral therapy and 579 to a largely ineffective combination therapy, and followed participants for 52 weeks. The target population was US people infected with HIV in 2006, as estimated by the Centers for Disease Control and Prevention. Results from the trial apply, albeit muted by 12%, to the target population, under the assumption that the authors have measured and correctly modeled the determinants of selection that reflect heterogeneity in the treatment effect. In simulations with a heterogeneous treatment effect, a conventional intent-to-treat estimate was biased with poor confidence limit coverage, but the proposed estimate was largely unbiased with appropriate confidence limit coverage. The proposed method standardizes observed trial results to a specified target population and thereby provides information regarding the generalizability of trial results.
Figures
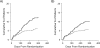
References
-
- Cole SR, Frangakis CE. The consistency statement in causal inference: a definition or an assumption? Epidemiology. 2009;20(1):3–5. - PubMed
-
- VanderWeele TJ. Concerning the consistency assumption in causal inference. Epidemiology. 2009;20(6):880–883. - PubMed
-
- Robins JM, Morgenstern H. The foundations of confounding in epidemiology. Comput Math Appl. 1987;14:869–916.

