Glucagon signaling modulates sweet taste responsiveness
- PMID: 20547661
- PMCID: PMC2996909
- DOI: 10.1096/fj.10-158105
Glucagon signaling modulates sweet taste responsiveness
Abstract
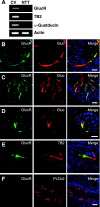
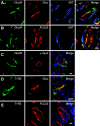
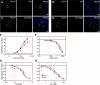
The gustatory system provides critical information about the quality and nutritional value of food before it is ingested. Thus, physiological mechanisms that modulate taste function in the context of nutritional needs or metabolic status could optimize ingestive decisions. We report that glucagon, which plays important roles in the maintenance of glucose homeostasis, enhances sweet taste responsiveness through local actions in the mouse gustatory epithelium. Using immunohistochemistry and confocal microscopy, we found that glucagon and its receptor (GlucR) are coexpressed in a subset of mouse taste receptor cells. Most of these cells also express the T1R3 taste receptor implicated in sweet and/or umami taste. Genetic or pharmacological disruption of glucagon signaling in behaving mice indicated a critical role for glucagon in the modulation of taste responsiveness. Scg5(-/-) mice, which lack mature glucagon, had significantly reduced responsiveness to sucrose as compared to wild-type littermates in brief-access taste tests. No significant differences were seen in responses to prototypical salty, sour, or bitter stimuli. Taste responsiveness to sucrose was similarly reduced upon acute and local disruption of glucagon signaling by the GlucR antagonist L-168,049. Together, these data indicate a role for local glucagon signaling in the peripheral modulation of sweet taste responsiveness.
Figures

Similar articles
-
Glucagon-like peptide-1 is specifically involved in sweet taste transmission.FASEB J. 2015 Jun;29(6):2268-80. doi: 10.1096/fj.14-265355. Epub 2015 Feb 12. FASEB J. 2015. PMID: 25678625 Free PMC article.
-
A subset of broadly responsive Type III taste cells contribute to the detection of bitter, sweet and umami stimuli.PLoS Genet. 2020 Aug 13;16(8):e1008925. doi: 10.1371/journal.pgen.1008925. eCollection 2020 Aug. PLoS Genet. 2020. PMID: 32790785 Free PMC article.
-
Gustatory stimuli representing different perceptual qualities elicit distinct patterns of neuropeptide secretion from taste buds.J Neurosci. 2013 Apr 24;33(17):7559-64. doi: 10.1523/JNEUROSCI.0372-13.2013. J Neurosci. 2013. Retraction in: J Neurosci. 2015 Jun 17;35(24):9248. doi: 10.1523/JNEUROSCI.1802-15.2015. PMID: 23616560 Free PMC article. Retracted.
-
Gustatory signaling in the periphery: detection, transmission, and modulation of taste information.Biol Pharm Bull. 2010;33(11):1772-7. doi: 10.1248/bpb.33.1772. Biol Pharm Bull. 2010. PMID: 21048297 Review.
-
Multiple receptor systems for glutamate detection in the taste organ.Biol Pharm Bull. 2008 Oct;31(10):1833-7. doi: 10.1248/bpb.31.1833. Biol Pharm Bull. 2008. PMID: 18827337 Review.
Cited by
-
Age-related changes in mouse taste bud morphology, hormone expression, and taste responsivity.J Gerontol A Biol Sci Med Sci. 2012 Apr;67(4):336-44. doi: 10.1093/gerona/glr192. Epub 2011 Nov 4. J Gerontol A Biol Sci Med Sci. 2012. PMID: 22056740 Free PMC article.
-
Y1 receptors modulate taste-related behavioral responsiveness in male mice to prototypical gustatory stimuli.Horm Behav. 2021 Nov;136:105056. doi: 10.1016/j.yhbeh.2021.105056. Epub 2021 Sep 9. Horm Behav. 2021. PMID: 34509673 Free PMC article.
-
Physiology of the tongue with emphasis on taste transduction.Physiol Rev. 2023 Apr 1;103(2):1193-1246. doi: 10.1152/physrev.00012.2022. Epub 2022 Nov 24. Physiol Rev. 2023. PMID: 36422992 Free PMC article. Review.
-
Longitudinal trajectories and determinants of human fungiform papillae density.Aging (Albany NY). 2021 Dec 2;13(23):24989-25003. doi: 10.18632/aging.203741. Epub 2021 Dec 2. Aging (Albany NY). 2021. PMID: 34857670 Free PMC article.
-
Lack of glucagon receptor signaling and its implications beyond glucose homeostasis.J Endocrinol. 2015 Mar;224(3):R123-30. doi: 10.1530/JOE-14-0614. Epub 2015 Jan 7. J Endocrinol. 2015. PMID: 25568163 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

