Subthreshold membrane potential oscillations in inferior olive neurons are dynamically regulated by P/Q- and T-type calcium channels: a study in mutant mice
- PMID: 20547676
- PMCID: PMC2956943
- DOI: 10.1113/jphysiol.2009.184705
Subthreshold membrane potential oscillations in inferior olive neurons are dynamically regulated by P/Q- and T-type calcium channels: a study in mutant mice
Abstract
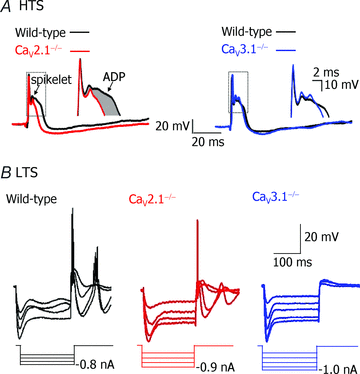
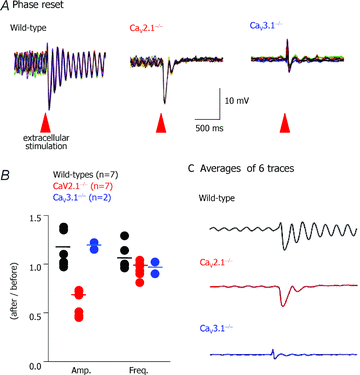
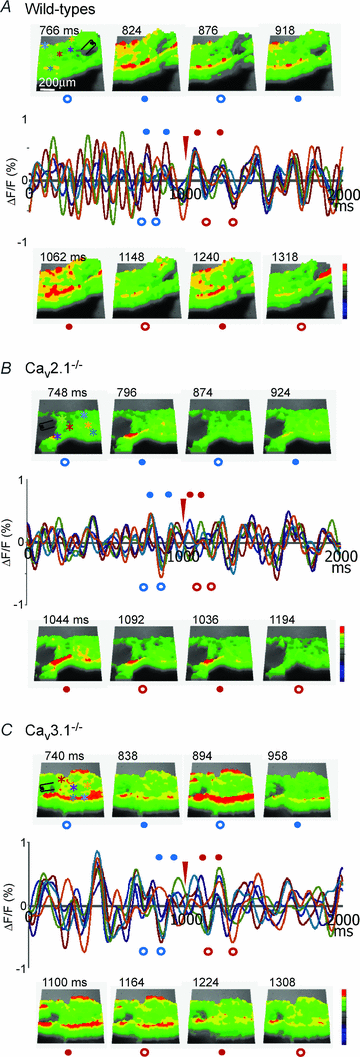
The role of P/Q- and T-type calcium channels in the rhythmic oscillatory behaviour of inferior olive (IO) neurons was investigated in mutant mice. Mice lacking either the CaV2.1 gene of the pore-forming alpha1A subunit for P/Q-type calcium channel, or the CaV3.1 gene of the pore-forming alpha1G subunit for T-type calcium channel were used. In vitro intracellular recording from IO neurons reveals that the amplitude and frequency of sinusoidal subthreshold oscillations (SSTOs) were reduced in the CaV2.1-/- mice. In the CaV3.1-/- mice, IO neurons also showed altered patterns of SSTOs and the probability of SSTO generation was significantly lower (15%, 5 of 34 neurons) than that of wild-type (78%, 31 of 40 neurons) or CaV2.1-/- mice (73%, 22 of 30 neurons). In addition, the low-threshold calcium spike and the sustained endogenous oscillation following rebound potentials were absent in IO neurons from CaV3.1-/- mice. Moreover, the phase-reset dynamics of oscillatory properties of single neurons and neuronal clusters in IO were remarkably altered in both CaV2.1-/- and CaV3.1-/- mice. These results suggest that both alpha1A P/Q- and alpha1G T-type calcium channels are required for the dynamic control of neuronal oscillations in the IO. These findings were supported by results from a mathematical IO neuronal model that incorporated T and P/Q channel kinetics.
Figures


References
-
- Bal T, McCormick DA. Synchronized oscillations in the inferior olive are controlled by the hyperpolarization-activated cation current Ih. J Neurophysiol. 1997;77:3145–3156. - PubMed
-
- Bell C, Kawasaki T. Relations among climbing fiber responses of nearby Purkinje cells. J Neurophysiol. 1972;35:155–169. - PubMed
-
- Benardo L, Foster R. Oscillatory behavior in inferior olive neurons: mechanism, modulation, cell aggregates. Brain Res Bull. 1986;17:773–784. - PubMed
-
- Bezrukov S, Vodyanoy I. Stochastic resonance and small-amplitude signal transduction in voltage-gated ion channels. In: Walleczek J, editor. Self-organized Biological Dynamics and Nonlinear Control. Cambridge: Cambridge University Press; 2000.
-
- Bulsara A, Hanggi P, Marchesoni F, Moss F, Shlesinger M. Special issue: Stochastic resonance in physics and biology. J Stat Phys. 1993;70:1–512.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

