In vivo expression of G-protein beta1gamma2 dimer in adult mouse skeletal muscle alters L-type calcium current and excitation-contraction coupling
- PMID: 20547679
- PMCID: PMC2956909
- DOI: 10.1113/jphysiol.2010.191593
In vivo expression of G-protein beta1gamma2 dimer in adult mouse skeletal muscle alters L-type calcium current and excitation-contraction coupling
Abstract
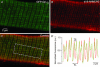
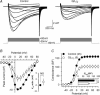
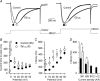
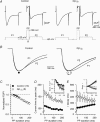
A number of G-protein-coupled receptors are expressed in skeletal muscle but their roles in muscle physiology and downstream effector systems remain poorly investigated. Here we explored the functional importance of the G-protein betagamma (Gbetagamma) signalling pathway on voltage-controlled Ca(2+) homeostasis in single isolated adult skeletal muscle fibres. A GFP-tagged Gbeta(1)gamma(2) dimer was expressed in vivo in mice muscle fibres. The GFP fluorescence pattern was consistent with a Gbeta(1)gamma(2) dimer localization in the transverse-tubule membrane. Membrane current and indo-1 fluorescence measurements performed under voltage-clamp conditions reveal a drastic reduction of both L-type Ca(2+) current density and of peak amplitude of the voltage-activated Ca(2+) transient in Gbeta(1)gamma(2)-expressing fibres. These effects were not observed upon expression of Gbeta(2)gamma(2), Gbeta(3)gamma(2) or Gbeta(4)gamma(2). Our data suggest that the G-protein beta(1)gamma(2) dimer may play an important regulatory role in skeletal muscle excitation-contraction coupling.
Figures


Similar articles
-
Rem inhibits skeletal muscle EC coupling by reducing the number of functional L-type Ca2+ channels.Biophys J. 2008 Apr 1;94(7):2631-8. doi: 10.1529/biophysj.107.116467. Epub 2008 Jan 11. Biophys J. 2008. PMID: 18192376 Free PMC article.
-
Excitation-contraction coupling in skeletal muscle of a mouse lacking the dihydropyridine receptor subunit gamma1.J Physiol. 2001 Jun 1;533(Pt 2):367-77. doi: 10.1111/j.1469-7793.2001.0367a.x. J Physiol. 2001. PMID: 11389198 Free PMC article.
-
Voltage sensor movements of CaV1.1 during an action potential in skeletal muscle fibers.Proc Natl Acad Sci U S A. 2021 Oct 5;118(40):e2026116118. doi: 10.1073/pnas.2026116118. Proc Natl Acad Sci U S A. 2021. PMID: 34583989 Free PMC article.
-
Excitation-contraction coupling from the 1950s into the new millennium.Clin Exp Pharmacol Physiol. 2006 Sep;33(9):763-72. doi: 10.1111/j.1440-1681.2006.04441.x. Clin Exp Pharmacol Physiol. 2006. PMID: 16922804 Review.
-
Functional equivalence of dihydropyridine receptor alpha1S and beta1a subunits in triggering excitation-contraction coupling in skeletal muscle.Biol Res. 2004;37(4):565-75. doi: 10.4067/s0716-97602004000400010. Biol Res. 2004. PMID: 15709683 Review.
Cited by
-
Inhibition of G-protein βγ signaling enhances T cell receptor-stimulated interleukin 2 transcription in CD4+ T helper cells.PLoS One. 2015 Jan 28;10(1):e0116575. doi: 10.1371/journal.pone.0116575. eCollection 2015. PLoS One. 2015. PMID: 25629163 Free PMC article.
-
Protein kinase C enhances plasma membrane expression of cardiac L-type calcium channel, CaV1.2.Channels (Austin). 2017 Nov 2;11(6):604-615. doi: 10.1080/19336950.2017.1369636. Epub 2017 Sep 21. Channels (Austin). 2017. PMID: 28901828 Free PMC article.
-
Pannexin-1 and CaV1.1 show reciprocal interaction during excitation-contraction and excitation-transcription coupling in skeletal muscle.J Gen Physiol. 2021 Dec 6;153(12):e202012635. doi: 10.1085/jgp.202012635. Epub 2021 Oct 12. J Gen Physiol. 2021. PMID: 34636893 Free PMC article.
-
Depression of voltage-activated Ca2+ release in skeletal muscle by activation of a voltage-sensing phosphatase.J Gen Physiol. 2015 Apr;145(4):315-30. doi: 10.1085/jgp.201411309. J Gen Physiol. 2015. PMID: 25825170 Free PMC article.
-
T-type calcium channels in synaptic plasticity.Channels (Austin). 2017 Mar 4;11(2):121-139. doi: 10.1080/19336950.2016.1238992. Epub 2016 Sep 21. Channels (Austin). 2017. PMID: 27653665 Free PMC article. Review.
References
-
- Agler HL, Evans J, Tay LH, Anderson MJ, Colecraft HM, Yue DT. G protein-gated inhibitory module of N-type Cav2.2 Ca2+ channels. Neuron. 2005;46:891–904. - PubMed
-
- Bannister RA. Bridging the myoplasmic gap: recent developments in skeletal muscle excitation-contraction coupling. J Muscle Res Cell Motil. 2007;28:275–283. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

