Allosteric ligands of the glucagon-like peptide 1 receptor (GLP-1R) differentially modulate endogenous and exogenous peptide responses in a pathway-selective manner: implications for drug screening
- PMID: 20547734
- PMCID: PMC3202488
- DOI: 10.1124/mol.110.065664
Allosteric ligands of the glucagon-like peptide 1 receptor (GLP-1R) differentially modulate endogenous and exogenous peptide responses in a pathway-selective manner: implications for drug screening
Abstract
The glucagon-like peptide-1 (GLP-1) receptor is a key regulator of insulin secretion and a major therapeutic target for treatment of diabetes. However, GLP-1 receptor function is complex, with multiple endogenous peptides that can interact with the receptor, including full-length (1-37) and truncated (7-37) forms of GLP-1 that can each exist in an amidated form and the related peptide oxyntomodulin. We have investigated two GLP-1 receptor allosteric modulators, Novo Nordisk compound 2 (6,7-dichloro2-methylsulfonyl-3-tert-butylaminoquinoxaline) and quercetin, and their ability to modify binding and signaling (cAMP formation, intracellular Ca(2+) mobilization, and extracellular signal-regulated kinase 1/2 phosphorylation) of each of the naturally occurring endogenous peptide agonists, as well as the clinically used peptide mimetic exendin-4. We identified and quantified stimulus bias across multiple endogenous peptides, with response profiles for truncated GLP-1 peptides distinct from those of either the full-length GLP-1 peptides or oxyntomodulin, the first demonstration of such behavior at the GLP-1 receptor. Compound 2 selectively augmented cAMP signaling but did so in a peptide-agonist dependent manner having greatest effect on oxyntomodulin, weaker effect on truncated GLP-1 peptides, and negligible effect on other peptide responses; these effects were principally driven by parallel changes in peptide agonist affinity. In contrast, quercetin selectively modulated calcium signaling but with effects only on truncated GLP-1 peptides or exendin and not oxyntomodulin or full-length peptides. These data have significant implications for how GLP-1 receptor targeted drugs are screened and developed, whereas the allosterically driven, agonist-selective, stimulus bias highlights the potential for distinct clinical efficacy depending on the properties of individual drugs.
Figures
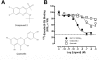

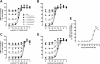
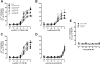
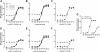
References
-
- Avlani V, May LT, Sexton PM, Christopoulos A. Application of a kinetic model to the apparently complex behavior of negative and positive allosteric modulators of muscarinic acetylcholine receptors. J Pharmacol Exp Ther. 2004;308:1062–1072. - PubMed
-
- Avlani VA, McLoughlin DJ, Sexton PM, Christopoulos A. The impact of orthosteric radioligand depletion on the quantification of allosteric modulator interactions. J Pharmacol Exp Ther. 2008;325:927–934. - PubMed
-
- Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131–2157. - PubMed
-
- Black J. Drugs from emasculated hormones: the principle of syntopic antagonism. Science. 1989;245:486–493. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

