Constitutive androstane receptor-mediated up-regulation of ATP-driven xenobiotic efflux transporters at the blood-brain barrier
- PMID: 20547735
- PMCID: PMC2939489
- DOI: 10.1124/mol.110.063685
Constitutive androstane receptor-mediated up-regulation of ATP-driven xenobiotic efflux transporters at the blood-brain barrier
Abstract
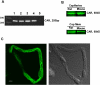
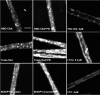
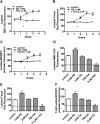
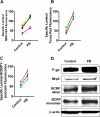
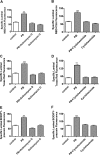
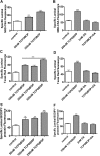
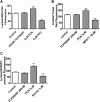
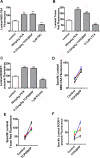
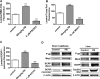
ATP-driven efflux transporters at the blood-brain barrier both protect against neurotoxicants and limit drug delivery to the brain. In other barrier and excretory tissues, efflux transporter expression is regulated by certain ligand-activated nuclear receptors. Here we identified constitutive androstane receptor (CAR) as a positive regulator of P-glycoprotein, multidrug resistance-associated protein 2 (Mrp2), and breast cancer resistance protein (BCRP) expression in rat and mouse brain capillaries. Exposing rat brain capillaries to the CAR activator, phenobarbital (PB), increased the transport activity and protein expression (Western blots) of P-glycoprotein, Mrp2, and BCRP. Induction of transport was abolished by the protein phosphatase 2A inhibitor, OA. Similar effects on transporter activity and expression were found when mouse brain capillaries were exposed to the mouse-specific CAR ligand, 1,4-bis-[2-(3,5-dichloropyridyloxy)]benzene (TCPOBOP). In brain capillaries from CAR-null mice, TCPOBOP did not increase transporter activity. Finally, treating mice with 0.33 mg/kg TCPOBOP or rats with 80 mg/kg PB increased P-glycoprotein-, Mrp2-, and BCRP-mediated transport and protein expression in brain capillaries assayed ex vivo. Thus, CAR activation selectively tightens the blood-brain barrier by increasing transport activity and protein expression of three xenobiotic efflux pumps.
Figures

References
-
- Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. (2010) Structure and function of the blood-brain barrier. Neurobiol Dis 37:13–25 - PubMed
-
- Akanuma S, Hori S, Ohtsuki S, Fujiyoshi M, Terasaki T. (2008) Expression of nuclear receptor mRNA and liver X receptor-mediated regulation of ABC transporter A1 at rat blood-brain barrier. Neurochem Int 52:669–674 - PubMed
-
- Allen JD, van Loevezijn A, Lakhai JM, van der Valk M, van Tellingen O, Reid G, Schellens JH, Koomen GJ, Schinkel AH. (2002) Potent and specific inhibition of the breast cancer resistance protein multidrug transporter in vitro and in mouse intestine by a novel analogue of fumitremorgin C. Mol Cancer Ther 1:417–425 - PubMed
-
- Anapolsky A, Teng S, Dixit S, Piquette-Miller M. (2006) The role of pregnane X receptor in 2-acetylaminofluorene-mediated induction of drug transport and -metabolizing enzymes in mice. Drug Metab Dispos 34:405–409 - PubMed
-
- Bauer B, Hartz AM, Fricker G, Miller DS. (2004) Pregnane X receptor up-regulation of P-glycoprotein expression and transport function at the blood-brain barrier. Mol Pharmacol 66:413–419 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

