Genome organization and pathogenicity of Corynebacterium diphtheriae C7(-) and PW8 strains
- PMID: 20547743
- PMCID: PMC2937438
- DOI: 10.1128/IAI.00049-10
Genome organization and pathogenicity of Corynebacterium diphtheriae C7(-) and PW8 strains
Abstract
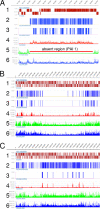
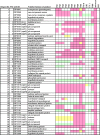
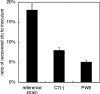
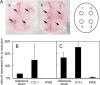
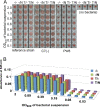
Corynebacterium diphtheriae is the causative agent of diphtheria. In 2003, the complete genomic nucleotide sequence of an isolate (NCTC13129) from a large outbreak in the former Soviet Union was published, in which the presence of 13 putative pathogenicity islands (PAIs) was demonstrated. In contrast, earlier work on diphtheria mainly employed the C7(-) strain for genetic analysis; therefore, current knowledge of the molecular genetics of the bacterium is limited to that strain. However, genomic information on the NCTC13129 strain has scarcely been compared to strain C7(-). Another important C. diphtheriae strain is Park-Williams no. 8 (PW8), which has been the only major strain used in toxoid vaccine production and for which genomic information also is not available. Here, we show by comparative genomic hybridization that at least 37 regions from the reference genome, including 11 of the 13 PAIs, are considered to be absent in the C7(-) genome. Despite this, the C7(-) strain still retained signs of pathogenicity, showing a degree of adhesion to Detroit 562 cells, as well as the formation of and persistence in abscesses in animal skin comparable to that of the NCTC13129 strain. In contrast, the PW8 strain, suggested to lack 14 genomic regions, including 3 PAIs, exhibited more reduced signs of pathogenicity. These results, together with great diversity in the presence of the 37 genomic regions among various C. diphtheriae strains shown by PCR analyses, suggest great heterogeneity of this pathogen, not only in genome organization, but also in pathogenicity.
Figures


Similar articles
-
Pangenomic study of Corynebacterium diphtheriae that provides insights into the genomic diversity of pathogenic isolates from cases of classical diphtheria, endocarditis, and pneumonia.J Bacteriol. 2012 Jun;194(12):3199-215. doi: 10.1128/JB.00183-12. Epub 2012 Apr 13. J Bacteriol. 2012. PMID: 22505676 Free PMC article.
-
Plasticity of Corynebacterium diphtheriae pathogenicity islands revealed by PCR.Genet Mol Res. 2011 Jun 28;10(2):1290-4. doi: 10.4238/vol10-2gmr1211. Genet Mol Res. 2011. PMID: 21732292
-
Evolution, epidemiology and diversity of Corynebacterium diphtheriae: New perspectives on an old foe.Infect Genet Evol. 2016 Sep;43:364-70. doi: 10.1016/j.meegid.2016.06.024. Epub 2016 Jun 9. Infect Genet Evol. 2016. PMID: 27291708 Review.
-
Genomic analysis of endemic clones of toxigenic and non-toxigenic Corynebacterium diphtheriae in Belarus during and after the major epidemic in 1990s.BMC Genomics. 2017 Nov 13;18(1):873. doi: 10.1186/s12864-017-4276-3. BMC Genomics. 2017. PMID: 29132312 Free PMC article.
-
Corynebacterium diphtheriae: genome diversity, population structure and genotyping perspectives.Infect Genet Evol. 2009 Jan;9(1):1-15. doi: 10.1016/j.meegid.2008.09.011. Epub 2008 Oct 19. Infect Genet Evol. 2009. PMID: 19007916 Review.
Cited by
-
Genome-wide comparison of Corynebacterium diphtheriae isolates from Australia identifies differences in the Pan-genomes between respiratory and cutaneous strains.BMC Genomics. 2018 Dec 4;19(1):869. doi: 10.1186/s12864-018-5147-2. BMC Genomics. 2018. PMID: 30509172 Free PMC article.
-
Pilus gene pool variation and the virulence of Corynebacterium diphtheriae clinical isolates during infection of a nematode.J Bacteriol. 2013 Aug;195(16):3774-83. doi: 10.1128/JB.00500-13. Epub 2013 Jun 14. J Bacteriol. 2013. PMID: 23772071 Free PMC article.
-
Molecular Characterization of Corynebacterium diphtheriae Outbreak Isolates, South Africa, March-June 2015.Emerg Infect Dis. 2017 Aug;23(8):1308-1315. doi: 10.3201/eid2308.162039. Emerg Infect Dis. 2017. PMID: 28726616 Free PMC article.
-
Next generation sequencing analysis of nine Corynebacterium ulcerans isolates reveals zoonotic transmission and a novel putative diphtheria toxin-encoding pathogenicity island.Genome Med. 2014 Nov 28;6(11):113. doi: 10.1186/s13073-014-0113-3. eCollection 2014. Genome Med. 2014. PMID: 25587356 Free PMC article.
-
Isolation and characterization of toxigenic Corynebacterium ulcerans from 2 closed colonies of cynomolgus macaques (Macaca fascicularis) in Japan.Comp Med. 2013 Jun;63(3):272-8. Comp Med. 2013. PMID: 23759530 Free PMC article.
References
-
- Albert, T. J., D. Dailidiene, G. Dailide, J. E. Norton, A. Kalia, T. A. Richmond, M. Molla, J. Singh, R. D. Green, and D. E. Berg. 2005. Mutation discovery in bacterial genomes: metronidazole resistance in Helicobacter pylori. Nat. Methods 2:951-953. - PubMed
-
- Barksdale, L., L. Garmise, and K. Horibata. 1960. Virulence, toxinogeny, and lysogeny in Corynebacterium diphtheriae. Ann. N. Y. Acad. Sci. 88:1093-1108. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

