Role of PknB kinase in antibiotic resistance and virulence in community-acquired methicillin-resistant Staphylococcus aureus strain USA300
- PMID: 20547748
- PMCID: PMC2916262
- DOI: 10.1128/IAI.00296-10
Role of PknB kinase in antibiotic resistance and virulence in community-acquired methicillin-resistant Staphylococcus aureus strain USA300
Abstract
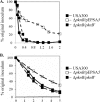
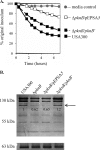
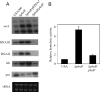
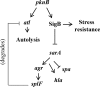
The regulation of cellular processes by eukaryote-like serine/threonine kinases is widespread in bacteria. In the last 2 years, several studies have examined the role of serine/threonine kinases in Staphylococcus aureus on cell wall metabolism, autolysis, and virulence, mostly in S. aureus laboratory isolates in the 8325-4 lineage. In this study, we showed that the pknB gene (also called stk1) of methicillin-resistant S. aureus (MRSA) strain COL and the community-acquired MRSA (CA-MRSA) strain USA300 is involved in cell wall metabolism, with the pknB mutant exhibiting enhanced sensitivity to beta-lactam antibiotics but not to other classes of antibiotics, including aminoglycosides, ciprofloxacin, bactrim, and other types of cell wall-active agents (e.g., vancomycin and bacitracin). Additionally, the pknB mutant of USA300 was found to be more resistant to Triton X-100-induced autolysis and also to lysis by lysostaphin. We also showed that pknB is a positive regulator of sigB activity, resulting in compromise in its response to heat and oxidative stresses. In association with reduced sigB activity, the expression levels of RNAII and RNAIII of agr and the downstream effector hla are upregulated while spa expression is downmodulated in the pknB mutant compared to the level in the parent. Consistent with an enhanced agr response in vitro, virulence studies of the pknB mutant of USA300 in a murine cutaneous model of infection showed that the mutant was more virulent than the parental strain. Collectively, our results have linked the pknB gene in CA-MRSA to antibiotic resistance, sigB activity, and virulence and have highlighted important differences in pknB phenotypes (virulence and sigB activity) between laboratory isolates and the prototypic CA-MRSA strain USA300.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

