Inactivation of Foxo3a and subsequent downregulation of PGC-1 alpha mediate nitric oxide-induced endothelial cell migration
- PMID: 20547753
- PMCID: PMC2916439
- DOI: 10.1128/MCB.00175-10
Inactivation of Foxo3a and subsequent downregulation of PGC-1 alpha mediate nitric oxide-induced endothelial cell migration
Abstract
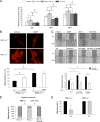
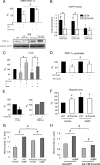
In damaged or proliferating endothelium, production of nitric oxide (NO) from endothelial nitric oxide synthase (eNOS) is associated with elevated levels of reactive oxygen species (ROS), which are necessary for endothelial migration. We aimed to elucidate the mechanism that mediates NO induction of endothelial migration. NO downregulates expression of peroxisome proliferator-activated receptor gamma coactivator 1 alpha (PGC-1 alpha), which positively modulates several genes involved in ROS detoxification. We tested whether NO-induced cell migration requires PGC-1 alpha downregulation and investigated the regulatory pathway involved. PGC-1 alpha negatively regulated NO-dependent endothelial cell migration in vitro, and inactivation of the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt) pathway, which is activated by NO, reduced NO-mediated downregulation of PGC-1 alpha. Expression of constitutively active Foxo3a, a target for Akt-mediated inactivation, reduced NO-dependent PGC-1 alpha downregulation. Foxo3a is also a direct transcriptional regulator of PGC-1 alpha, and we found that a functional FoxO binding site in the PGC-1 alpha promoter is also a NO response element. These results show that NO-mediated downregulation of PGC-1 alpha is necessary for NO-induced endothelial migration and that NO/protein kinase G (PKG)-dependent downregulation of PGC-1 alpha and the ROS detoxification system in endothelial cells are mediated by the PI3K/Akt signaling pathway and subsequent inactivation of the FoxO transcription factor Foxo3a.
Figures




References
-
- Borniquel, S., I. Valle, S. Cadenas, S. Lamas, and M. Monsalve. 2006. Nitric oxide regulates mitochondrial oxidative stress protection via the transcriptional coactivator PGC-1alpha. FASEB J. 20:1889-1891. - PubMed
-
- Daitoku, H., K. Yamagata, H. Matsuzaki, M. Hatta, and A. Fukamizu. 2003. Regulation of PGC-1 promoter activity by protein kinase B and the forkhead transcription factor FKHR. Diabetes 52:642-649. - PubMed
-
- Dimmeler, S., and A. M. Zeiher. 1999. Nitric oxide—an endothelial cell survival factor. Cell Death Differ. 6:964-968. - PubMed
-
- Dudzinski, D. M., J. Igarashi, D. Greif, and T. Michel. 2006. The regulation and pharmacology of endothelial nitric oxide synthase. Annu. Rev. Pharmacol. Toxicol. 46:235-276. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
