Regulation of the Rho family small GTPase Wrch-1/RhoU by C-terminal tyrosine phosphorylation requires Src
- PMID: 20547754
- PMCID: PMC2937548
- DOI: 10.1128/MCB.01646-09
Regulation of the Rho family small GTPase Wrch-1/RhoU by C-terminal tyrosine phosphorylation requires Src
Abstract
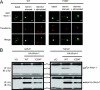
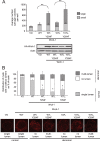
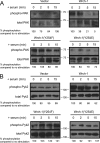
Wrch-1 is an atypical Rho family small GTPase with roles in migration, epithelial cell morphogenesis, osteoclastogenesis, and oncogenic transformation. Here, we observed rapid relocalization of Wrch-1 from the plasma membrane upon serum stimulation. Studies revealed a requirement for serum-stimulated tyrosine phosphorylation of Wrch-1 at residue Y254 within its C-terminal membrane targeting domain, mediated by the nonreceptor tyrosine kinase Src. Genetic or pharmacological loss of Src kinase activity blocked both phosphorylation and relocalization of Wrch-1. Functionally, Y254 was required for proper Wrch-1 modulation of cystogenesis in three-dimensional culture, and the phospho-deficient mutant, Y254F, was enhanced in Wrch-1-mediated anchorage-independent growth. Mechanistically, C-terminal tyrosine phosphorylation and subsequent relocalization of Wrch-1 downregulated its ability to interact with and activate its effectors by decreasing active Wrch-1-GTP, perhaps by altering proximity to a GEF or GAP. Phospho-deficient Wrch-1(Y254F) remained at the plasma membrane and GTP bound and continued to recruit and activate its effector PAK, even upon serum stimulation. In contrast, a phospho-mimetic mutant, Y254E, was constitutively endosomally localized and GDP bound and failed to recruit PAK unless mutated to be constitutively active/GAP insensitive. C-terminal tyrosine phosphorylation thus represents a new paradigm in posttranslational control of small GTPase localization, activation, and biological function.
Figures






References
-
- Adamson, P., C. J. Marshall, A. Hall, and P. A. Tilbrook. 1992. Post-translational modifications of p21rho proteins. J. Biol. Chem. 267:20033-20038. - PubMed
-
- Aspenstrom, P., A. Ruusala, and D. Pacholsky. 2007. Taking Rho GTPases to the next level: the cellular functions of atypical Rho GTPases. Exp. Cell Res. 313:3673-3679. - PubMed
-
- Berzat, A. C., D. C. Brady, J. J. Fiordalisi, and A. D. Cox. 2006. Using inhibitors of prenylation to block localization and transforming activity. Methods Enzymol. 407:575-597. - PubMed
-
- Berzat, A. C., J. E. Buss, E. J. Chenette, C. A. Weinbaum, A. Shutes, C. J. Der, A. Minden, and A. D. Cox. 2005. Transforming activity of the Rho family GTPase, Wrch-1, a Wnt-regulated Cdc42 homolog, is dependent on a novel carboxyl-terminal palmitoylation motif. J. Biol. Chem. 280:33055-33065. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
