Aerosol therapy with colistin methanesulfonate: a biopharmaceutical issue illustrated in rats
- PMID: 20547787
- PMCID: PMC2934989
- DOI: 10.1128/AAC.00411-10
Aerosol therapy with colistin methanesulfonate: a biopharmaceutical issue illustrated in rats
Abstract
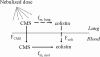
The aim of this study was to evaluate the biopharmaceutical behavior of colistin methanesulfonate (CMS) with special focus on colistin presystemic formation after CMS nebulization in rats. CMS was administered (15 mg x kg(-1) of body weight) either intravenously for systemic pharmacokinetic studies (n = 6) or as an intratracheal nebulization for systemic pharmacokinetic studies (n = 5) or for CMS and colistin concentration measurements in epithelial lining fluid (ELF) at 30, 120, and 240 min after nebulization (n = 14). CMS and colistin concentrations were determined by a new liquid chromatography (LC)-tandem mass spectrometry (MS/MS) assay. Pharmacokinetic parameters were estimated by noncompartmental analysis. CMS and colistin pharmacokinetic data were consistent with previously published values when comparisons were possible. The fraction of the CMS dose converted systematically into colistin after intravenous CMS administration was estimated to be 12.5% on average. After CMS nebulization it was estimated that about two-thirds of the dose was directly absorbed within the systemic circulation, whereas one-third was first converted into active colistin, which was eventually absorbed. As a consequence, the colistin area under curve (AUC) reflecting systemic availability was about 4-fold greater after CMS intratracheal nebulization (607 +/- 240 microg x min x ml(-1)) than after CMS intravenous administration (160 +/- 20 microg x min x ml(-1)). CMS concentrations in ELF at 30 min and 120 min postnebulization were very high (in the order of several mg/ml) due to the limited volume of ELF but were considerably reduced at 240 min. Although lower (15% +/- 5% at 120 min) in relative terms, colistin concentrations in ELF could be high enough for being active against microorganisms following CMS nebulization.
Figures

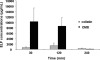
Similar articles
-
Substantial Targeting Advantage Achieved by Pulmonary Administration of Colistin Methanesulfonate in a Large-Animal Model.Antimicrob Agents Chemother. 2016 Dec 27;61(1):e01934-16. doi: 10.1128/AAC.01934-16. Print 2017 Jan. Antimicrob Agents Chemother. 2016. PMID: 27821445 Free PMC article.
-
Comparison of intrapulmonary and systemic pharmacokinetics of colistin methanesulfonate (CMS) and colistin after aerosol delivery and intravenous administration of CMS in critically ill patients.Antimicrob Agents Chemother. 2014 Dec;58(12):7331-9. doi: 10.1128/AAC.03510-14. Epub 2014 Sep 29. Antimicrob Agents Chemother. 2014. PMID: 25267660 Free PMC article.
-
Population pharmacokinetics of colistin methanesulfonate in rats: achieving sustained lung concentrations of colistin for targeting respiratory infections.Antimicrob Agents Chemother. 2013 Oct;57(10):5087-95. doi: 10.1128/AAC.01127-13. Epub 2013 Aug 5. Antimicrob Agents Chemother. 2013. PMID: 23917323 Free PMC article.
-
Colistin in critically ill patients.Minerva Anestesiol. 2013 Feb;79(2):200-8. Epub 2012 Dec 17. Minerva Anestesiol. 2013. PMID: 23241733 Review.
-
Clinical Pharmacokinetics, Pharmacodynamics and Toxicodynamics of Polymyxins: Implications for Therapeutic Use.Adv Exp Med Biol. 2019;1145:219-249. doi: 10.1007/978-3-030-16373-0_15. Adv Exp Med Biol. 2019. PMID: 31364081 Review.
Cited by
-
Pulmonary Pharmacokinetics of Oseltamivir Carboxylate in Rats after Nebulization or Intravenous Administration of Its Prodrug, Oseltamivir Phosphate.Antimicrob Agents Chemother. 2019 May 24;63(6):e00074-19. doi: 10.1128/AAC.00074-19. Print 2019 Jun. Antimicrob Agents Chemother. 2019. PMID: 30962337 Free PMC article.
-
Biopharmaceutical characterization of nebulized antimicrobial agents in rats: 1. Ciprofloxacin, moxifloxacin, and grepafloxacin.Antimicrob Agents Chemother. 2014 Jul;58(7):3942-9. doi: 10.1128/AAC.02818-14. Epub 2014 May 5. Antimicrob Agents Chemother. 2014. PMID: 24798283 Free PMC article.
-
Clinical Pharmacokinetics and Pharmacodynamics of Colistin.Clin Pharmacokinet. 2017 Dec;56(12):1441-1460. doi: 10.1007/s40262-017-0561-1. Clin Pharmacokinet. 2017. PMID: 28550595 Review.
-
Biopharmaceutical Characterization of Nebulized Antimicrobial Agents in Rats: 6. Aminoglycosides.Antimicrob Agents Chemother. 2018 Sep 24;62(10):e01261-18. doi: 10.1128/AAC.01261-18. Print 2018 Oct. Antimicrob Agents Chemother. 2018. PMID: 30082284 Free PMC article.
-
Aerosolized Polymyxin B for Treatment of Respiratory Tract Infections: Determination of Pharmacokinetic-Pharmacodynamic Indices for Aerosolized Polymyxin B against Pseudomonas aeruginosa in a Mouse Lung Infection Model.Antimicrob Agents Chemother. 2017 Jul 25;61(8):e00211-17. doi: 10.1128/AAC.00211-17. Print 2017 Aug. Antimicrob Agents Chemother. 2017. PMID: 28559256 Free PMC article.
References
-
- Berlana, D., J. M. Llop, E. Fort, M. B. Badia, and R. Jodar. 2005. Use of colistin in the treatment of multiple-drug-resistant Gram-negative infections. Am. J. Health Syst. Pharm. 62:39-47. - PubMed
-
- Chandenier, J., S. Bernard, J. Montharu, E. Bailly, F. Fetissof, M. de Monte, G. Desoubeaux, P. Diot, and D. Richard-Lenoble. 2009. The utility of a nebulised intra-tracheal rat model of invasive pulmonary aspergillosis. Mycoses 52:239-245. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

