Aerosol therapy with colistin methanesulfonate: a biopharmaceutical issue illustrated in rats
- PMID: 20547787
- PMCID: PMC2934989
- DOI: 10.1128/AAC.00411-10
Aerosol therapy with colistin methanesulfonate: a biopharmaceutical issue illustrated in rats
Abstract
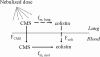
The aim of this study was to evaluate the biopharmaceutical behavior of colistin methanesulfonate (CMS) with special focus on colistin presystemic formation after CMS nebulization in rats. CMS was administered (15 mg x kg(-1) of body weight) either intravenously for systemic pharmacokinetic studies (n = 6) or as an intratracheal nebulization for systemic pharmacokinetic studies (n = 5) or for CMS and colistin concentration measurements in epithelial lining fluid (ELF) at 30, 120, and 240 min after nebulization (n = 14). CMS and colistin concentrations were determined by a new liquid chromatography (LC)-tandem mass spectrometry (MS/MS) assay. Pharmacokinetic parameters were estimated by noncompartmental analysis. CMS and colistin pharmacokinetic data were consistent with previously published values when comparisons were possible. The fraction of the CMS dose converted systematically into colistin after intravenous CMS administration was estimated to be 12.5% on average. After CMS nebulization it was estimated that about two-thirds of the dose was directly absorbed within the systemic circulation, whereas one-third was first converted into active colistin, which was eventually absorbed. As a consequence, the colistin area under curve (AUC) reflecting systemic availability was about 4-fold greater after CMS intratracheal nebulization (607 +/- 240 microg x min x ml(-1)) than after CMS intravenous administration (160 +/- 20 microg x min x ml(-1)). CMS concentrations in ELF at 30 min and 120 min postnebulization were very high (in the order of several mg/ml) due to the limited volume of ELF but were considerably reduced at 240 min. Although lower (15% +/- 5% at 120 min) in relative terms, colistin concentrations in ELF could be high enough for being active against microorganisms following CMS nebulization.
Figures

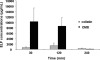
References
-
- Berlana, D., J. M. Llop, E. Fort, M. B. Badia, and R. Jodar. 2005. Use of colistin in the treatment of multiple-drug-resistant Gram-negative infections. Am. J. Health Syst. Pharm. 62:39-47. - PubMed
-
- Chandenier, J., S. Bernard, J. Montharu, E. Bailly, F. Fetissof, M. de Monte, G. Desoubeaux, P. Diot, and D. Richard-Lenoble. 2009. The utility of a nebulised intra-tracheal rat model of invasive pulmonary aspergillosis. Mycoses 52:239-245. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

