Structure-activity analysis of vinylogous urea inhibitors of human immunodeficiency virus-encoded ribonuclease H
- PMID: 20547794
- PMCID: PMC2935023
- DOI: 10.1128/AAC.00434-10
Structure-activity analysis of vinylogous urea inhibitors of human immunodeficiency virus-encoded ribonuclease H
Abstract
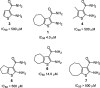
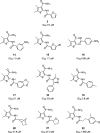
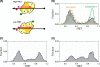
Vinylogous ureas 2-amino-5,6,7,8-tetrahydro-4H-cyclohepta[b]thiophene-3-carboxamide and N-[3-(aminocarbonyl)-4,5-dimethyl-2-thienyl]-2-furancarboxamide (compounds 1 and 2, respectively) were recently identified to be modestly potent inhibitors of the RNase H activity of HIV-1 and HIV-2 reverse transcriptase (RT). Both compounds shared a 3-CONH(2)-substituted thiophene ring but were otherwise structurally unrelated, which prevented a precise definition of the pharmacophore. We have therefore examined a larger series of vinylogous ureas carrying amide, amine, and cycloalkane modifications of the thiophene ring of compound 1. While cycloheptane- and cyclohexane-substituted derivatives retained potency, cyclopentane and cyclooctane substitutions eliminated activity. In the presence of a cycloheptane ring, modifying the 2-NH(2) or 3-CONH(2) functions decreased the potency. With respect to compound 2, vinylogous ureas whose dimethylthiophene ring contained modifications of the 2-NH(2) and 3-CONH(2) functions were investigated. 2-NH(2)-modified analogs displayed potency equivalent to or enhanced over that of compound 2, the most active of which, compound 16, reflected intramolecular cyclization of the 2-NH(2) and 3-CONH(2) groups. Molecular modeling was used to define an inhibitor binding site in the p51 thumb subdomain, suggesting that an interaction with the catalytically conserved His539 of the p66 RNase H domain could underlie inhibition of RNase H activity. Collectively, our data indicate that multiple functional groups of vinylogous ureas contribute to their potencies as RNase H inhibitors. Finally, single-molecule spectroscopy indicates that vinylogous ureas have the property of altering the reverse transcriptase orientation on a model RNA-DNA hybrid mimicking initiation plus-strand DNA synthesis.
Figures





References
-
- Beard, W. A., S. J. Stahl, H. R. Kim, K. Bebenek, A. Kumar, M. P. Strub, S. P. Becerra, T. A. Kunkel, and S. H. Wilson. 1994. Structure/function studies of human immunodeficiency virus type 1 reverse transcriptase. Alanine scanning mutagenesis of an alpha-helix in the thumb subdomain. J. Biol. Chem. 269:28091-28097. - PubMed
-
- Bonache, M. C., E. Quesada, C. W. Sheen, J. Balzarini, N. Sluis-Cremer, M. J. Perez-Perez, M. J. Camarasa, and A. San-Felix. 2008. Novel N-3 substituted TSAO-T derivatives: synthesis and anti-HIV-evaluation. Nucleosides Nucleotides Nucleic Acids 27:351-367. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Medical

