Discovery of potent small-molecule inhibitors of multidrug-resistant Plasmodium falciparum using a novel miniaturized high-throughput luciferase-based assay
- PMID: 20547797
- PMCID: PMC2934977
- DOI: 10.1128/AAC.00431-10
Discovery of potent small-molecule inhibitors of multidrug-resistant Plasmodium falciparum using a novel miniaturized high-throughput luciferase-based assay
Abstract
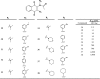
Malaria is a global health problem that causes significant mortality and morbidity, with more than 1 million deaths per year caused by Plasmodium falciparum. Most antimalarial drugs face decreased efficacy due to the emergence of resistant parasites, which necessitates the discovery of new drugs. To identify new antimalarials, we developed an automated 384-well plate screening assay using P. falciparum parasites that stably express cytoplasmic firefly luciferase. After initial optimization, we tested two different types of compound libraries: known bioactive collections (Library of Pharmacologically Active Compounds [LOPAC] and the library from the National Institute of Neurological Disorders and Stroke [NINDS]) and a library of uncharacterized compounds (ChemBridge). A total of 12,320 compounds were screened at 5.5 microM. Selecting only compounds that reduced parasite growth by 85% resulted in 33 hits from the combined bioactive collection and 130 hits from the ChemBridge library. Fifteen novel drug-like compounds from the bioactive collection were found to be active against P. falciparum. Twelve new chemical scaffolds were found from the ChemBridge hits, the most potent of which was a series based on the 1,4-naphthoquinone scaffold, which is structurally similar to the FDA-approved antimalarial atovaquone. However, in contrast to atovaquone, which acts to inhibit the bc(1) complex and block the electron transport chain in parasite mitochondria, we have determined that our new 1,4-napthoquinones act in a novel, non-bc(1)-dependent mechanism and remain potent against atovaquone- and chloroquine-resistant parasites. Ultimately, this study may provide new probes to understand the molecular details of the malaria life cycle and to identify new antimalarials.
Figures




References
-
- Biagini, G. A., N. Fisher, N. Berry, P. A. Stocks, B. Meunier, D. P. Williams, R. Bonar-Law, P. G. Bray, A. Owen, P. M. O'Neill, and S. A. Ward. 2008. Acridinediones: selective and potent inhibitors of the malaria parasite mitochondrial bc1 complex. Mol. Pharmacol. 73:1347-1355. - PubMed
-
- Burckhalter, J. H., F. H. Tendick, E. M. Jones, P. A. Jones, W. F. Holcomb, and A. L. Rawlins. 1948. Aminoalkylphenols as antimalarials. II. (Heterocyclic-amino)-α-amino-o-cresols. The synthesis of camoquin. J. Am. Chem. Soc. 70:1363-1373. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

