Generic vancomycin products fail in vivo despite being pharmaceutical equivalents of the innovator
- PMID: 20547818
- PMCID: PMC2916296
- DOI: 10.1128/AAC.01044-09
Generic vancomycin products fail in vivo despite being pharmaceutical equivalents of the innovator
Abstract
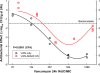
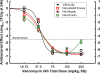
Generic versions of intravenous antibiotics are not required to demonstrate therapeutic equivalence with the innovator because therapeutic equivalence is assumed from pharmaceutical equivalence. To test such assumptions, we studied three generic versions of vancomycin in simultaneous experiments with the innovator and determined the concentration and potency of the active pharmaceutical ingredient by microbiological assay, single-dose pharmacokinetics in infected mice, antibacterial effect by broth microdilution and time-kill curves (TKC), and pharmacodynamics against two wild-type strains of Staphylococcus aureus by using the neutropenic mouse thigh infection model. The main outcome measure was the comparison of magnitudes and patterns of in vivo efficacy between generic products and the innovator. Except for one product exhibiting slightly greater concentration, vancomycin generics were undistinguishable from the innovator based on concentration and potency, protein binding, in vitro antibacterial effect determined by minimal inhibitory or bactericidal concentrations and TKC, and serum pharmacokinetics. Despite such similarities, all generic products failed in vivo to kill S. aureus, while the innovator displayed the expected bactericidal efficacy: maximum antibacterial effect (Emax) (95% confidence interval [CI]) was 2.04 (1.89 to 2.19), 2.59 (2.21 to 2.98), and 3.48 (2.92 to 4.04) versus 5.65 (5.52 to 5.78) log10 CFU/g for three generics and the innovator product, respectively (P<0.0001, any comparison). Nonlinear regression analysis suggests that generic versions of vancomycin contain inhibitory and stimulatory principles within their formulations that cause agonistic-antagonistic actions responsible for in vivo failure. In conclusion, pharmaceutical equivalence does not imply therapeutic equivalence for vancomycin.
Figures
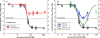

Comment in
-
In vivo inferiority of generic product compared with branded vancomycin: a paradigm shift.Ther Drug Monit. 2012 Feb;34(1):2-3. doi: 10.1097/FTD.0b013e318243e739. Ther Drug Monit. 2012. PMID: 22210101 No abstract available.
Similar articles
-
Comparison of In Vivo Pharmacokinetics and Pharmacodynamics of Vancomycin Products Available in Korea.Yonsei Med J. 2020 Apr;61(4):301-309. doi: 10.3349/ymj.2020.61.4.301. Yonsei Med J. 2020. PMID: 32233172 Free PMC article.
-
In vitro and in vivo comparison of the anti-staphylococcal efficacy of generic products and the innovator of oxacillin.BMC Infect Dis. 2010 Jun 4;10:153. doi: 10.1186/1471-2334-10-153. BMC Infect Dis. 2010. PMID: 20525378 Free PMC article.
-
Therapeutic equivalence requires pharmaceutical, pharmacokinetic, and pharmacodynamic identities: true bioequivalence of a generic product of intravenous metronidazole.Antimicrob Agents Chemother. 2012 May;56(5):2659-65. doi: 10.1128/AAC.06012-11. Epub 2012 Feb 13. Antimicrob Agents Chemother. 2012. PMID: 22330928 Free PMC article.
-
The role of vancomycin in the treatment paradigm.Clin Infect Dis. 2006 Jan 1;42 Suppl 1:S51-7. doi: 10.1086/491714. Clin Infect Dis. 2006. PMID: 16323121 Review.
-
Susceptibility relationship between vancomycin and daptomycin in Staphylococcus aureus: facts and assumptions.Lancet Infect Dis. 2009 Oct;9(10):617-24. doi: 10.1016/S1473-3099(09)70200-2. Lancet Infect Dis. 2009. PMID: 19778764 Review.
Cited by
-
Comparative in vitro study of the antimicrobial activities of different commercial antibiotic products of vancomycin.BMC Clin Pharmacol. 2011 Jul 21;11:9. doi: 10.1186/1472-6904-11-9. BMC Clin Pharmacol. 2011. PMID: 21777438 Free PMC article.
-
Myths and Misconceptions around Antibiotic Resistance: Time to Get Rid of Them.Infect Chemother. 2022 Sep;54(3):393-408. doi: 10.3947/ic.2022.0060. Epub 2022 Aug 5. Infect Chemother. 2022. PMID: 36047302 Free PMC article. Review.
-
Pharmacodynamic evaluation of the activities of six parenteral vancomycin products available in the United States.Antimicrob Agents Chemother. 2015 Jan;59(1):622-32. doi: 10.1128/AAC.03710-14. Epub 2014 Nov 10. Antimicrob Agents Chemother. 2015. PMID: 25385113 Free PMC article.
-
Intrawound vancomycin to prevent infections after spine surgery: a systematic review and meta-analysis.Eur Spine J. 2015 Mar;24(3):533-42. doi: 10.1007/s00586-014-3357-0. Epub 2014 May 18. Eur Spine J. 2015. PMID: 24838506
-
Knowledge, attitudes and practice survey about antimicrobial resistance and prescribing among physicians in a hospital setting in Lima, Peru.BMC Clin Pharmacol. 2011 Nov 15;11:18. doi: 10.1186/1472-6904-11-18. BMC Clin Pharmacol. 2011. PMID: 22085536 Free PMC article.
References
-
- Adelman, C. C., and J. Norris. 2001. Usefulness of foreign aid for health care in less-developed countries. Lancet 358:2174. - PubMed
-
- Bauer, J., S. Spanton, R. Henry, J. Quick, W. Dziki, W. Porter, and J. Morris. 2001. Ritonavir: an extraordinary example of conformational polymorphism. Pharm. Res. 18:859-866. - PubMed
-
- Beam, T. R., Jr., D. N. Gilbert, and C. M. Kunin. 1992. General guidelines for the clinical evaluation of anti-infective drug products. Infectious Diseases Society of America and the Food and Drug Administration. Clin. Infect. Dis. 15(Suppl. 1):S5-S32. - PubMed
-
- Best, G. K., N. H. Best, and N. N. Durham. 1968. Chromatographic separation of the vancomycin complex. Antimicrob. Agents Chemother. 8:115-119. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

