Pathogen-specific regulatory T cells delay the arrival of effector T cells in the lung during early tuberculosis
- PMID: 20547826
- PMCID: PMC2901066
- DOI: 10.1084/jem.20091885
Pathogen-specific regulatory T cells delay the arrival of effector T cells in the lung during early tuberculosis
Abstract
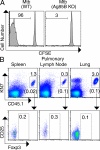
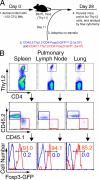
The ability of the adaptive immune system to restrict Mycobacterium tuberculosis (Mtb) is impeded by activated Foxp3(+) regulatory T (T reg) cells. The importance of pathogen-specific T reg cells in this process has not been addressed. We show that T reg cell expansion after aerosol Mtb infection does not occur until Mtb is transported to the pulmonary lymph node (pLN), and Mtb-specific T reg cells have an increased propensity to proliferate. Even small numbers of Mtb-specific T reg cells are capable of delaying the priming of effector CD4(+) and CD8(+) T cells in the pLN and their subsequent accumulation in the lung, the primary site of infection. This delay likely prolongs the initial phase of bacterial expansion and explains the higher bacterial burden observed in these mice. Thus, T reg cells recognizing Mtb-derived antigens specifically and potently restrict protective immune responses during tuberculosis.
Figures





References
-
- Antunes I., Tolaini M., Kissenpfennig A., Iwashiro M., Kuribayashi K., Malissen B., Hasenkrug K., Kassiotis G. 2008. Retrovirus-specificity of regulatory T cells is neither present nor required in preventing retrovirus-induced bone marrow immune pathology. Immunity. 29:782–794 10.1016/j.immuni.2008.09.016 - DOI - PMC - PubMed
-
- Ariga H., Shimohakamada Y., Nakada M., Tokunaga T., Kikuchi T., Kariyone A., Tamura T., Takatsu K. 2007. Instruction of naive CD4+ T-cell fate to T-bet expression and T helper 1 development: roles of T-cell receptor-mediated signals. Immunology. 122:210–221 10.1111/j.1365-2567.2007.02630.x - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

