Early Miocene hippopotamids (Cetartiodactyla) constrain the phylogenetic and spatiotemporal settings of hippopotamid origin
- PMID: 20547829
- PMCID: PMC2900691
- DOI: 10.1073/pnas.1001373107
Early Miocene hippopotamids (Cetartiodactyla) constrain the phylogenetic and spatiotemporal settings of hippopotamid origin
Abstract
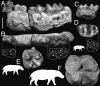
The affinities of the Hippopotamidae are at the core of the phylogeny of Cetartiodactyla (even-toed mammals: cetaceans, ruminants, camels, suoids, and hippos). Molecular phylogenies support Cetacea as sister group of the Hippopotamidae, implying a long ghost lineage between the earliest cetaceans (approximately 53 Ma) and the earliest hippopotamids (approximately 16 Ma). Morphological studies have proposed two different sister taxa for hippopotamids: suoids (notably palaeochoerids) or anthracotheriids. Evaluating these phylogenetic hypotheses requires substantiating the poorly known early history of the Hippopotamidae. Here, we undertake an original morphological phylogenetic analysis including several "suiform" families and previously unexamined early Miocene taxa to test previous conflicting hypotheses. According to our results, Morotochoerus ugandensis and Kulutherium rusingensis, until now regarded as the sole African palaeochoerid and the sole African bunodont anthracotheriid, respectively, are unambiguously included within the Hippopotamidae. They are the earliest known hippopotamids and set the family fossil record back to the early Miocene (approximately 21 Ma). The analysis reveals that hippopotamids displayed an unsuspected taxonomic and body size diversity and remained restricted to Africa during most of their history, until the latest Miocene. Our results also confirm the deep nesting of Hippopotamidae within the paraphyletic Anthracotheriidae; this finding allows us to reconstruct the sequence of dental innovations that links advanced selenodont anthracotheriids to hippopotamids, previously a source of major disagreements on hippopotamid origins. The analysis demonstrates a close relationship between Eocene choeropotamids and anthracotheriids, a relationship that potentially fills the evolutionary gap between earliest hippopotamids and cetaceans implied by molecular analyses.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

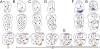

Similar articles
-
The position of Hippopotamidae within Cetartiodactyla.Proc Natl Acad Sci U S A. 2005 Feb 1;102(5):1537-41. doi: 10.1073/pnas.0409518102. Epub 2005 Jan 26. Proc Natl Acad Sci U S A. 2005. PMID: 15677331 Free PMC article.
-
Evolving between land and water: key questions on the emergence and history of the Hippopotamidae (Hippopotamoidea, Cetancodonta, Cetartiodactyla).Biol Rev Camb Philos Soc. 2011 Aug;86(3):601-25. doi: 10.1111/j.1469-185X.2010.00162.x. Epub 2010 Oct 15. Biol Rev Camb Philos Soc. 2011. PMID: 20946539 Review.
-
Hippos stem from the longest sequence of terrestrial cetartiodactyl evolution in Africa.Nat Commun. 2015 Feb 24;6:6264. doi: 10.1038/ncomms7264. Nat Commun. 2015. PMID: 25710445
-
Hippopotamidae (Cetartiodactyla, Hippopotamoidea) from Kanapoi, Kenya, and the taxonomic status of the late early Pliocene hippopotamids from the Turkana Basin.J Hum Evol. 2020 Mar;140:102377. doi: 10.1016/j.jhevol.2017.07.017. Epub 2017 Sep 29. J Hum Evol. 2020. PMID: 28966046
-
A complete phylogeny of the whales, dolphins and even-toed hoofed mammals (Cetartiodactyla).Biol Rev Camb Philos Soc. 2005 Aug;80(3):445-73. doi: 10.1017/s1464793105006743. Biol Rev Camb Philos Soc. 2005. PMID: 16094808 Review.
Cited by
-
A phylogenomic analysis of the role and timing of molecular adaptation in the aquatic transition of cetartiodactyl mammals.R Soc Open Sci. 2015 Sep 30;2(9):150156. doi: 10.1098/rsos.150156. eCollection 2015 Sep. R Soc Open Sci. 2015. PMID: 26473040 Free PMC article.
-
Paleobiogeographical origins of Fasciola hepatica and F. gigantica in light of new DNA sequence characteristics of F. nyanzae from hippopotamus.Front Vet Sci. 2022 Sep 9;9:990872. doi: 10.3389/fvets.2022.990872. eCollection 2022. Front Vet Sci. 2022. PMID: 36157179 Free PMC article.
-
The historical biogeography of Mammalia.Philos Trans R Soc Lond B Biol Sci. 2011 Sep 12;366(1577):2478-502. doi: 10.1098/rstb.2011.0023. Philos Trans R Soc Lond B Biol Sci. 2011. PMID: 21807730 Free PMC article. Review.
-
Eighteen million years of diverse enamel proteomes from the East African Rift.Nature. 2025 Jul;643(8072):712-718. doi: 10.1038/s41586-025-09040-9. Epub 2025 Jul 9. Nature. 2025. PMID: 40634615 Free PMC article.
-
Virtual endocranial cast of earliest Eocene Diacodexis (Artiodactyla, Mammalia) and morphological diversity of early artiodactyl brains.Proc Biol Sci. 2012 Sep 22;279(1743):3670-7. doi: 10.1098/rspb.2012.1156. Epub 2012 Jul 4. Proc Biol Sci. 2012. PMID: 22764165 Free PMC article.
References
-
- Arnason U, Gullberg A, Gretarsdottir S, Ursing B, Janke A. The mitochondrial genome of the sperm whale and a new molecular reference for estimating eutherian divergence dates. J Mol Evol. 2000;50:569–578. - PubMed
-
- Gatesy J. More DNA support for a Cetacea/Hippopotamidae clade: The blood-clotting protein gene gamma-fibrinogen. Mol Biol Evol. 1997;14:537–543. - PubMed
-
- Gatesy J. Molecular evidence for the phylogenetic affinities of Cetacea. In: Thewissen JGM, editor. The Emergence of Whales. New York: Plenum; 1998. pp. 63–111.
-
- Irwin DM, Arnason U. Cytochrome b gene of marine mammals: Phylogeny and evolution. J Mamm Evol. 1994;2:37–55.
-
- Marcot JD. Molecular phylogeny of terrestrial artiodactyls: Conflicts and resolution. In: Prothero DR, Foss SE, editors. The Evolution of Artiodactyls. Baltimore: The Johns Hopkins Univ Press; 2007. pp. 4–18.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

