Fgf-9 is required for angiogenesis and osteogenesis in long bone repair
- PMID: 20547837
- PMCID: PMC2900703
- DOI: 10.1073/pnas.1003317107
Fgf-9 is required for angiogenesis and osteogenesis in long bone repair
Abstract
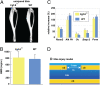
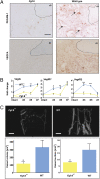
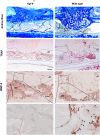
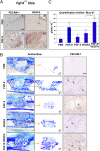
Bone healing requires a complex interaction of growth factors that establishes an environment for efficient bone regeneration. Among these, FGFs have been considered important for intrinsic bone-healing capacity. In this study, we analyzed the role of Fgf-9 in long bone repair. One-millimeter unicortical defects were created in tibias of Fgf-9(+/-) and wild-type mice. Histomorphometry revealed that half-dose gene of Fgf-9 markedly reduced bone regeneration as compared with wild-type. Both immunohistochemistry and RT-PCR analysis revealed markedly decreased levels of proliferating cell nuclear antigen (PCNA), Runt-related transcription factor 2 (Runx2), osteocalcin, Vega-a, and platelet endothelial cell adhesion molecule 1 (PECAM-1) in Fgf-9(+/-) defects. muCT angiography indicated dramatic impairment of neovascularization in Fgf-9(+/-) mice as compared with controls. Treatment with FGF-9 protein promoted angiogenesis and successfully rescued the healing capacity of Fgf-9(+/-) mice. Importantly, although other pro-osteogenic factors [Fgf-2, Fgf-18, and bone morphogenic protein 2 (Bmp-2)] still were present in Fgf-9(+/-) mice, they could not compensate for the haploinsufficiency of the Fgf-9 gene. Therefore, endogenous Fgf-9 seems to play an important role in long bone repair. Taken together our data suggest a unique role for Fgf-9 in bone healing, presumably by initiating angiogenesis through Vegf-a. Moreover, this study further supports the embryonic phenotype previously observed in the developing limb, thus promoting the concept that healing processes in adult organisms may recapitulate embryonic skeletal development.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Einhorn TA. The cell and molecular biology of fracture healing. Clin Orthop Relat Res. 1998;46(355, Suppl):S7–S21. - PubMed
-
- Gerstenfeld LC, Cullinane DM, Barnes GL, Graves DT, Einhorn TA. Fracture healing as a post-natal developmental process: Molecular, spatial, and temporal aspects of its regulation. J Cell Biochem. 2003;88:873–884. - PubMed
-
- Schindeler A, McDonald MM, Bokko P, Little DG. Bone remodeling during fracture repair: The cellular picture. Semin Cell Dev Biol. 2008;19:459–466. - PubMed
-
- Eswarakumar VP, et al. The IIIc alternative of Fgfr2 is a positive regulator of bone formation. Development. 2002;129:3783–3793. - PubMed
-
- Deng C, Wynshaw-Boris A, Zhou F, Kuo A, Leder P. Fibroblast growth factor receptor 3 is a negative regulator of bone growth. Cell. 1996;84:911–921. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

