Parkin overexpression selects against a deleterious mtDNA mutation in heteroplasmic cybrid cells
- PMID: 20547844
- PMCID: PMC2900690
- DOI: 10.1073/pnas.0914569107
Parkin overexpression selects against a deleterious mtDNA mutation in heteroplasmic cybrid cells
Abstract
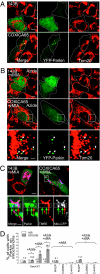
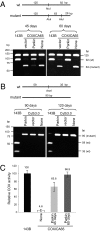
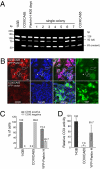
Mitochondrial genomes with deleterious mutations can replicate in cells along with wild-type genomes in a state of heteroplasmy, and are a cause of severe inherited syndromes, such as mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke (MELAS), neuropathy, ataxia, retinitis pigmentosa-maternally inherited Leigh syndrome (NARP-MILS), and Leber's hereditary optic neuropathy (LHON). The cytosolic E3 ligase, Parkin, commonly mutated in recessive familial parkinsonism, translocates to depolarized mitochondria and induces their autophagic elimination, suggesting that Parkin may signal the selective removal of defective mitochondria within the cell. We report that long-term overexpression of Parkin can eliminate mitochondria with deleterious COXI mutations in heteroplasmic cybrid cells, thereby enriching cells for wild-type mtDNA and restoring cytochrome c oxidase activity. After relieving cybrid cells of Parkin overexpression, a more favorable wild-type to mutant mitochondrial genome ratio is stably maintained. These data support the model that Parkin functions in a mitochondrial quality control pathway. Additionally, they suggest that transiently increasing levels of Parkin expression might ameliorate certain mitochondrial diseases.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Kraytsberg Y, et al. Mitochondrial DNA deletions are abundant and cause functional impairment in aged human substantia nigra neurons. Nat Genet. 2006;38:518–520. - PubMed
-
- Bender A, et al. High levels of mitochondrial DNA deletions in substantia nigra neurons in aging and Parkinson disease. Nat Genet. 2006;38:515–517. - PubMed
-
- Schapira AH. Mitochondria in the aetiology and pathogenesis of Parkinson's disease. Lancet Neurol. 2008;7:97–109. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

