Success or failure of vaccination for HPV16-positive vulvar lesions correlates with kinetics and phenotype of induced T-cell responses
- PMID: 20547850
- PMCID: PMC2900675
- DOI: 10.1073/pnas.1006500107
Success or failure of vaccination for HPV16-positive vulvar lesions correlates with kinetics and phenotype of induced T-cell responses
Abstract
One half of a group of 20 patients with human papillomavirus type 16 (HPV16)-induced vulvar intraepithelial neoplasia grade 3 displayed a complete regression (CR) after therapeutic vaccination with HPV16 E6/E7 synthetic long peptides. Patients with relatively larger lesions generally did not display a CR. To investigate immune correlates of treatment failure, patients were grouped according to median lesion size at study entry, and HPV16-specific immunity was analyzed at different time points by complementary immunological assays. The group of patients with smaller lesions displayed stronger and broader vaccine-prompted HPV16-specific proliferative responses with higher IFNgamma (P = 0.0003) and IL-5 (P < 0.0001) levels than patients with large lesions. Characteristically, this response was accompanied by a distinct peak in cytokine levels after the first vaccination. In contrast, the patient group with larger lesions mounted higher frequencies of HPV16-specific CD4(+)CD25(+)Foxp3(+) T cells (P = 0.005) and displayed a lower HPV16-specific IFNgamma/IL-10 ratio after vaccination (P < 0.01). No disparity in T memory immunity to control antigens was found, indicating that the differences in HPV-specific immunity did not reflect general immune failure. We observed a strong correlation between a defined set of vaccine-prompted specific immune responses and the clinical efficacy of therapeutic vaccination. Notably, a high ratio of HPV16-specific vaccine-prompted effector T cells to HPV16-specific CD4(+)CD25(+)Foxp3(+) T cells was predictive of clinical success. Foxp3(+) T cells have been associated previously with impaired immunity in malignancies. Here we demonstrate that the vaccine-prompted level of this population is associated with early treatment failure.
Conflict of interest statement
Conflict of interest statement: This study has been conducted by the Leiden University Medical Center (LUMC), which holds a patent on the use of synthetic long peptides as vaccine (US 7.202.034). C.J.M.M. and S.H.v.d.B. are named as inventors on this patent. The LUMC does not share the financial benefit from this patent with its employees. C.J. M. M. has been employed part-time (75%) since January 20, 2008, by ISA Pharmaceuticals, which exploits this long-peptide vaccine patent, and has been granted options on ISA Pharmaceuticals stock.
Figures
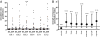
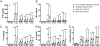
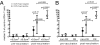
References
-
- Weaver BA. Epidemiology and natural history of genital human papillomavirus infection. J Am Osteopath Assoc. 2006;106(3, Suppl 1):S2–S8. - PubMed
-
- Wheeler CM. Natural history of human papillomavirus infections, cytologic and histologic abnormalities, and cancer. Obstet Gynecol Clin North Am. 2008;35:519–536, vii. - PubMed
-
- Bosch FX, et al. Epidemiology and natural history of human papillomavirus infections and type-specific implications in cervical neoplasia. Vaccine. 2008;26(Suppl 10):K1–K16. - PubMed
-
- Walboomers JM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–19. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

