Comparison of natamycin and voriconazole for the treatment of fungal keratitis
- PMID: 20547942
- PMCID: PMC3774126
- DOI: 10.1001/archophthalmol.2010.102
Comparison of natamycin and voriconazole for the treatment of fungal keratitis
Abstract
Objective: To conduct a therapeutic exploratory clinical trial comparing clinical outcomes of treatment with topical natamycin vs topical voriconazole for fungal keratitis.
Methods: The multicenter, double-masked, clinical trial included 120 patients with fungal keratitis at Aravind Eye Hospital in India who were randomized to receive either topical natamycin or topical voriconazole and either had repeated scraping of the epithelium or not.
Main outcome measures: The primary outcome was best spectacle-corrected visual acuity (BSCVA) at 3 months. Other outcomes included scar size, perforations, and a subanalysis of BSCVA at 3 months in patients with an enrollment visual acuity of 20/40 to 20/400.
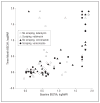
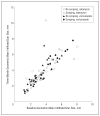
Results: Compared with those who received natamycin, voriconazole-treated patients had an approximately 1-line improvement in BSCVA at 3 months after adjusting for scraping in a multivariate regression model but the difference was not statistically significant (P = .29). Scar size at 3 months was slightly greater with voriconazole after adjusting for scraping (P = .48). Corneal perforations in the voriconazole group (10 of 60 patients) were not significantly different than in the natamycin-treated group (9 of 60 patients) (P >.99). Scraping was associated with worse BSCVA at 3 months after adjusting for drug (P = .06). Patients with baseline BSCVA of 20/40 to 20/400 showed a trend toward a 2-line improvement in visual acuity with voriconazole (P = .07).
Conclusions: Overall, there were no significant differences in visual acuity, scar size, and perforations between voriconazole- and natamycin-treated patients. There was a trend toward scraping being associated with worse outcomes. Application to Clinical Practice The benefit seen with voriconazole in the subgroup of patients with baseline visual acuity of 20/40 to 20/400 needs to be validated in a confirmatory clinical trial. Trial Registration clinicaltrials.gov Identifier: NCT00557362.
Figures


Comment in
-
Natamycin and voriconazole in fungal keratitis.Arch Ophthalmol. 2011 Jun;129(6):814; author reply 814-5. doi: 10.1001/archophthalmol.2011.97. Arch Ophthalmol. 2011. PMID: 21670358 No abstract available.
References
-
- Alfonso EC, Cantu-Dibildox J, Munir WM, et al. Insurgence of Fusarium keratitis associated with contact lens wear. Arch Ophthalmol. 2006;124(7):941–947. - PubMed
-
- Rosa RH, Jr, Miller D, Alfonso EC. The changing spectrum of fungal keratitis in south Florida. Ophthalmology. 1994;101(6):1005–1013. - PubMed
-
- Xie L, Zhong W, Shi W, Sun S. Spectrum of fungal keratitis in north China. Ophthalmology. 2006;113(11):1943–1948. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

