Brain microglial cytokines in neurogenic hypertension
- PMID: 20547972
- PMCID: PMC2929640
- DOI: 10.1161/HYPERTENSIONAHA.110.150409
Brain microglial cytokines in neurogenic hypertension
Abstract
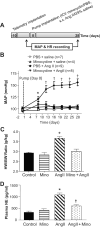
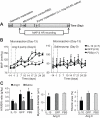
Accumulating evidence indicates a key role of inflammation in hypertension and cardiovascular disorders. However, the role of inflammatory processes in neurogenic hypertension remains to be determined. Thus, our objective in the present study was to test the hypothesis that activation of microglial cells and the generation of proinflammatory cytokines in the paraventricular nucleus (PVN) contribute to neurogenic hypertension. Intracerebroventricular infusion of minocycline, an anti-inflammatory antibiotic, caused a significant attenuation of mean arterial pressure, cardiac hypertrophy, and plasma norepinephrine induced by chronic angiotensin II infusion. This was associated with decreases in the numbers of activated microglia and mRNAs for interleukin (IL) 1beta, IL-6, and tumor necrosis factor-alpha, and an increase in the mRNA for IL-10 in the PVN. Overexpression of IL-10 induced by recombinant adenoassociated virus-mediated gene transfer in the PVN mimicked the antihypertensive effects of minocycline. Furthermore, acute application of a proinflammatory cytokine, IL-1beta, into the left ventricle or the PVN in normal rats resulted in a significant increase in mean arterial pressure. Collectively, this indicates that angiotensin II induced hypertension involves activation of microglia and increases in proinflammatory cytokines in the PVN. These data have significant implications on the development of innovative therapeutic strategies for the control of neurogenic hypertension.
Figures
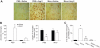



Similar articles
-
Minocycline and Pyrrolidine Dithiocarbamate Attenuate Hypertension via Suppressing Activation of Microglia in the Hypothalamic Paraventricular Nucleus.Tohoku J Exp Med. 2023 Jan 27;259(2):163-172. doi: 10.1620/tjem.2022.J102. Epub 2022 Dec 1. Tohoku J Exp Med. 2023. PMID: 36450479
-
Blockade of Microglial Activation in Hypothalamic Paraventricular Nucleus Improves High Salt-Induced Hypertension.Am J Hypertens. 2022 Sep 1;35(9):820-827. doi: 10.1093/ajh/hpac052. Am J Hypertens. 2022. PMID: 35439285
-
Inhibition of microglial activation in rats attenuates paraventricular nucleus inflammation in Gαi2 protein-dependent, salt-sensitive hypertension.Exp Physiol. 2019 Dec;104(12):1892-1910. doi: 10.1113/EP087924. Epub 2019 Oct 20. Exp Physiol. 2019. PMID: 31631436 Free PMC article.
-
Chronic infusion of enalaprilat into hypothalamic paraventricular nucleus attenuates angiotensin II-induced hypertension and cardiac hypertrophy by restoring neurotransmitters and cytokines.Toxicol Appl Pharmacol. 2014 Feb 1;274(3):436-44. doi: 10.1016/j.taap.2013.12.001. Epub 2013 Dec 14. Toxicol Appl Pharmacol. 2014. PMID: 24342267
-
ACE2 overexpression in the paraventricular nucleus attenuates angiotensin II-induced hypertension.Cardiovasc Res. 2011 Dec 1;92(3):401-8. doi: 10.1093/cvr/cvr242. Epub 2011 Sep 27. Cardiovasc Res. 2011. PMID: 21952934 Free PMC article.
Cited by
-
J-shaped associations of pan-immune-inflammation value and systemic inflammation response index with stroke among American adults with hypertension: evidence from NHANES 1999-2020.Front Neurol. 2024 Jul 31;15:1417863. doi: 10.3389/fneur.2024.1417863. eCollection 2024. Front Neurol. 2024. PMID: 39144717 Free PMC article.
-
Bidirectional neuro-glial signaling modalities in the hypothalamus: role in neurohumoral regulation.Auton Neurosci. 2013 Apr;175(1-2):51-60. doi: 10.1016/j.autneu.2012.12.009. Epub 2013 Jan 30. Auton Neurosci. 2013. PMID: 23375650 Free PMC article. Review.
-
Brain-mediated dysregulation of the bone marrow activity in angiotensin II-induced hypertension.Hypertension. 2012 Nov;60(5):1316-23. doi: 10.1161/HYPERTENSIONAHA.112.199547. Epub 2012 Oct 8. Hypertension. 2012. PMID: 23045460 Free PMC article.
-
Central Inhibition of Tumor Necrosis Factor Alpha Reduces Hypertension by Attenuating Oxidative Stress in the Rostral Ventrolateral Medulla in Renovascular Hypertensive Rats.Front Physiol. 2019 Apr 30;10:491. doi: 10.3389/fphys.2019.00491. eCollection 2019. Front Physiol. 2019. PMID: 31114507 Free PMC article.
-
The gut microbiota and the brain-gut-kidney axis in hypertension and chronic kidney disease.Nat Rev Nephrol. 2018 Jul;14(7):442-456. doi: 10.1038/s41581-018-0018-2. Nat Rev Nephrol. 2018. PMID: 29760448 Free PMC article. Review.
References
-
- Ferrario CM, Strawn WB. Role of the renin-angiotensin-aldosterone system and proinflammatory mediators in cardiovascular disease. Am J Cardiol. 2006;98:121–128. - PubMed
-
- Schillaci G, Pirro M, Gemelli F, Pasqualini L, Vaudo G, Marchesi S, Siepi D, Bagaglia F, Mannarino E. Increased C-reactive protein concentrations in never-treated hypertension: The role of systolic and pulse pressures. J Hypertens. 2003;21:1841–1846. - PubMed
-
- Stumpf C, John S, Jukic J, Yilmaz A, Raaz D, Schmieder RE, Daniel WG, Garlichs CD. Enhanced levels of platelet P-selectin and circulating cytokines in young patients with mild arterial hypertension. J Hypertens. 2005;23:995–1000. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

