Membrane potential-dependent inactivation of voltage-gated ion channels in alpha-cells inhibits glucagon secretion from human islets
- PMID: 20547976
- PMCID: PMC2927942
- DOI: 10.2337/db09-1505
Membrane potential-dependent inactivation of voltage-gated ion channels in alpha-cells inhibits glucagon secretion from human islets
Abstract
Objective: To document the properties of the voltage-gated ion channels in human pancreatic alpha-cells and their role in glucagon release.
Research design and methods: Glucagon release was measured from intact islets. [Ca(2+)](i) was recorded in cells showing spontaneous activity at 1 mmol/l glucose. Membrane currents and potential were measured by whole-cell patch-clamping in isolated alpha-cells identified by immunocytochemistry.
Result: Glucose inhibited glucagon secretion from human islets; maximal inhibition was observed at 6 mmol/l glucose. Glucagon secretion at 1 mmol/l glucose was inhibited by insulin but not by ZnCl(2). Glucose remained inhibitory in the presence of ZnCl(2) and after blockade of type-2 somatostatin receptors. Human alpha-cells are electrically active at 1 mmol/l glucose. Inhibition of K(ATP)-channels with tolbutamide depolarized alpha-cells by 10 mV and reduced the action potential amplitude. Human alpha-cells contain heteropodatoxin-sensitive A-type K(+)-channels, stromatoxin-sensitive delayed rectifying K(+)-channels, tetrodotoxin-sensitive Na(+)-currents, and low-threshold T-type, isradipine-sensitive L-type, and omega-agatoxin-sensitive P/Q-type Ca(2+)-channels. Glucagon secretion at 1 mmol/l glucose was inhibited by 40-70% by tetrodotoxin, heteropodatoxin-2, stromatoxin, omega-agatoxin, and isradipine. The [Ca(2+)](i) oscillations depend principally on Ca(2+)-influx via L-type Ca(2+)-channels. Capacitance measurements revealed a rapid (<50 ms) component of exocytosis. Exocytosis was negligible at voltages below -20 mV and peaked at 0 mV. Blocking P/Q-type Ca(2+)-currents abolished depolarization-evoked exocytosis.
Conclusions: Human alpha-cells are electrically excitable, and blockade of any ion channel involved in action potential depolarization or repolarization results in inhibition of glucagon secretion. We propose that voltage-dependent inactivation of these channels underlies the inhibition of glucagon secretion by tolbutamide and glucose.
Figures
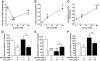




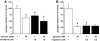

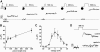
Similar articles
-
Somatostatin release, electrical activity, membrane currents and exocytosis in human pancreatic delta cells.Diabetologia. 2009 Aug;52(8):1566-78. doi: 10.1007/s00125-009-1382-z. Epub 2009 May 14. Diabetologia. 2009. PMID: 19440689
-
Voltage-gated ion channels in human pancreatic beta-cells: electrophysiological characterization and role in insulin secretion.Diabetes. 2008 Jun;57(6):1618-28. doi: 10.2337/db07-0991. Epub 2008 Apr 4. Diabetes. 2008. PMID: 18390794
-
ATP-sensitive K+ channel-dependent regulation of glucagon release and electrical activity by glucose in wild-type and SUR1-/- mouse alpha-cells.Diabetes. 2004 Dec;53 Suppl 3:S181-9. doi: 10.2337/diabetes.53.suppl_3.s181. Diabetes. 2004. PMID: 15561909
-
'Resistance is futile?' - paradoxical inhibitory effects of KATP channel closure in glucagon-secreting α-cells.J Physiol. 2020 Nov;598(21):4765-4780. doi: 10.1113/JP279775. Epub 2020 Aug 7. J Physiol. 2020. PMID: 32716554 Free PMC article. Review.
-
K(ATP)-channels and glucose-regulated glucagon secretion.Trends Endocrinol Metab. 2008 Oct;19(8):277-84. doi: 10.1016/j.tem.2008.07.003. Epub 2008 Sep 2. Trends Endocrinol Metab. 2008. PMID: 18771934 Review.
Cited by
-
In situ electrophysiological examination of pancreatic α cells in the streptozotocin-induced diabetes model, revealing the cellular basis of glucagon hypersecretion.Diabetes. 2013 Feb;62(2):519-30. doi: 10.2337/db11-0786. Epub 2012 Oct 5. Diabetes. 2013. PMID: 23043159 Free PMC article.
-
Modeling the Amino Acid Effect on Glucagon Secretion from Pancreatic Alpha Cells.Metabolites. 2022 Apr 13;12(4):348. doi: 10.3390/metabo12040348. Metabolites. 2022. PMID: 35448534 Free PMC article.
-
ATP-regulated potassium channels and voltage-gated calcium channels in pancreatic alpha and beta cells: similar functions but reciprocal effects on secretion.Diabetologia. 2014 Sep;57(9):1749-61. doi: 10.1007/s00125-014-3279-8. Epub 2014 Jun 7. Diabetologia. 2014. PMID: 24906950 Review.
-
Islet Function in the Pathogenesis of Cystic Fibrosis-Related Diabetes Mellitus.Clin Med Insights Endocrinol Diabetes. 2021 Jul 13;14:11795514211031204. doi: 10.1177/11795514211031204. eCollection 2021. Clin Med Insights Endocrinol Diabetes. 2021. PMID: 34345195 Free PMC article. Review.
-
Role of KATP channels in glucose-regulated glucagon secretion and impaired counterregulation in type 2 diabetes.Cell Metab. 2013 Dec 3;18(6):871-82. doi: 10.1016/j.cmet.2013.10.014. Cell Metab. 2013. PMID: 24315372 Free PMC article.
References
-
- Cryer PE. Hypoglycaemia: the limiting factor in the glycaemic management of Type I and Type II diabetes. Diabetologia 2002;45:937–948 - PubMed
-
- Lefebvre PJ. (Ed.). Glucagon and Diabetes. In Handbook of Experimental Pharmacology. Berlin, Springer, 1996, p. 115–131
-
- Gromada J, Franklin I, Wollheim CB. Alpha-cells of the endocrine pancreas: 35 years of research but the enigma remains. Endocr Rev 2007;28:84–116 - PubMed
-
- Olofsson CS, Salehi A, Gopel SO, Holm C, Rorsman P. Palmitate stimulation of glucagon secretion in mouse pancreatic alpha-cells results from activation of L-type calcium channels and elevation of cytoplasmic calcium. Diabetes 2004;53:2836–2843 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

