The two C-terminal tyrosines stabilize occluded Na/K pump conformations containing Na or K ions
- PMID: 20548052
- PMCID: PMC2894553
- DOI: 10.1085/jgp.201010407
The two C-terminal tyrosines stabilize occluded Na/K pump conformations containing Na or K ions
Abstract
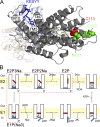
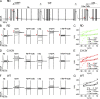
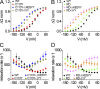
Interactions of the three transported Na ions with the Na/K pump remain incompletely understood. Na/K pump crystal structures show that the extended C terminus of the Na,K-adenosine triphosphatase (ATPase) alpha subunit directly contacts transmembrane helices. Deletion of the last five residues (KETYY in almost all Na/K pumps) markedly lowered the apparent affinity for Na activation of pump phosphorylation from ATP, a reflection of cytoplasmic Na affinity for forming the occluded E1P(Na3) conformation. ATPase assays further suggested that C-terminal truncations also interfere with low affinity Na interactions, which are attributable to extracellular effects. Because extracellular Na ions traverse part of the membrane's electric field to reach their binding sites in the Na/K pump, their movements generate currents that can be monitored with high resolution. We report here electrical measurements to examine how Na/K pump interactions with extracellular Na ions are influenced by C-terminal truncations. We deleted the last two (YY) or five (KESYY) residues in Xenopus laevis alpha1 Na/K pumps made ouabain resistant by either of two kinds of point mutations and measured their currents as 10-mM ouabain-sensitive currents in Xenopus oocytes after silencing endogenous Xenopus Na/K pumps with 1 microM ouabain. We found the low affinity inhibitory influence of extracellular Na on outward Na/K pump current at negative voltages to be impaired in all of the C-terminally truncated pumps. Correspondingly, voltage jump-induced transient charge movements that reflect pump interactions with extracellular Na ions were strongly shifted to more negative potentials; this signals a several-fold reduction of the apparent affinity for extracellular Na in the truncated pumps. Parallel lowering of Na affinity on both sides of the membrane argues that the C-terminal contacts provide important stabilization of the occluded E1P(Na3) conformation, regardless of the route of Na ion entry into the binding pocket. Gating measurements of palytoxin-opened Na/K pump channels additionally imply that the C-terminal contacts also help stabilize pump conformations with occluded K ions.
Figures
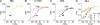




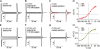


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

