Attenuated progression of diet-induced steatohepatitis in glutathione-deficient mice
- PMID: 20548286
- PMCID: PMC3156113
- DOI: 10.1038/labinvest.2010.112
Attenuated progression of diet-induced steatohepatitis in glutathione-deficient mice
Abstract
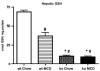
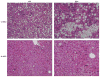
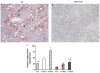
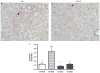
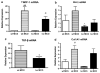
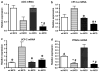
In nonalcoholic fatty liver disease (NAFLD), depletion of hepatic antioxidants may contribute to the progression of steatosis to nonalcoholic steatohepatitis (NASH) by increasing oxidative stress that produces lipid peroxidation, inflammation, and fibrosis. We investigated whether depletion of glutathione (GSH) increases NASH-associated hepatic pathology in mice fed a diet deficient in methionine and choline (MCD diet). Wild-type (wt) mice and genetically GSH-deficient mice lacking the modifier subunit of glutamate cysteine ligase (Gclm null mice), the rate-limiting enzyme for de novo synthesis of GSH, were fed the MCD diet, a methionine/choline-sufficient diet, or standard chow for 21 days. We assessed NASH-associated hepatic pathology, including steatosis, fibrosis, inflammation, and hepatocyte ballooning, and used the NAFLD Scoring System to evaluate the extent of changes. We measured triglyceride levels, determined the level of lipid peroxidation products, and measured by qPCR the expression of mRNAs for several proteins associated with lipid metabolism, oxidative stress, and fibrosis. MCD-fed GSH-deficient Gclm null mice were to a large extent protected from MCD diet-induced excessive fat accumulation, hepatocyte injury, inflammation, and fibrosis. Compared with wt animals, MCD-fed Gclm null mice had much lower levels of F₂-isoprostanes, lower expression of acyl-CoA oxidase, carnitine palmitoyltransferase 1a, uncoupling protein-2, stearoyl-coenzyme A desaturase-1, transforming growth factor-β, and plasminogen activator inhibitor-1 mRNAs, and higher activity of catalase, indicative of low oxidative stress, inhibition of triglyceride synthesis, and lower expression of profibrotic proteins. Global gene analysis of hepatic RNA showed that compared with wt mice, the livers of Gclm null mice have a high capacity to metabolize endogenous and exogenous compounds, have lower levels of lipogenic proteins, and increased antioxidant activity. Thus, metabolic adaptations resulting from severe GSH deficiency seem to protect against the development of steatohepatitis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
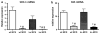

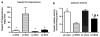
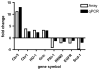
References
-
- Delgado JS. Evolving trends in nonalcoholic fatty liver disease. Eur J Intern Med. 2008;19:75–82. - PubMed
-
- Chitturi S, Farrell GC, Hashimoto E, et al. Non-alcoholic fatty liver disease in the Asia-Pacific region: definitions and overview of proposed guidelines. J Gastroenterol Hepatol. 2007;22:778–787. - PubMed
-
- Clark JM, Diehl AM. Nonalcoholic fatty liver disease: an underrecognized cause of cryptogenic cirrhosis. JAMA. 2003;289:3000–3004. - PubMed
-
- Cortez-Pinto H, de Moura MC, Day CP. Non-alcoholic steatohepatitis: from cell biology to clinical practice. J Hepatol. 2006;44:197–208. - PubMed
-
- Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology. 2006;43(2 Suppl 1):S99–S112. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U19ES011387/ES/NIEHS NIH HHS/United States
- R37CA023226/CA/NCI NIH HHS/United States
- R01CA127228/CA/NCI NIH HHS/United States
- R01 CA074131/CA/NCI NIH HHS/United States
- T32 ES007032/ES/NIEHS NIH HHS/United States
- T32ES007032/ES/NIEHS NIH HHS/United States
- R01 CA127228/CA/NCI NIH HHS/United States
- R01ES10849/ES/NIEHS NIH HHS/United States
- R01CA074131/CA/NCI NIH HHS/United States
- R37 CA023226/CA/NCI NIH HHS/United States
- P30ES07033/ES/NIEHS NIH HHS/United States
- U19 ES011387/ES/NIEHS NIH HHS/United States
- P30 ES007033/ES/NIEHS NIH HHS/United States
- R01 ES010849/ES/NIEHS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

