The Efficacy and Safety of Long-term Oral Cyclosporine Treatment for Patients with Atopic Dermatitis
- PMID: 20548874
- PMCID: PMC2883409
- DOI: 10.5021/ad.2010.22.1.9
The Efficacy and Safety of Long-term Oral Cyclosporine Treatment for Patients with Atopic Dermatitis
Abstract
Background: Steroids are used in conventional treatment of atopic dermatitis (AD) and they are very effective for improving the symptoms, but they also have several complications. Many studies have reported that short-term use of cyclosporine (CsA) is effective for severe AD as a substitute for steroid. However, there are very few studies on the long-term use of CsA for AD in the Korean population.
Objective: The purpose of this study was to investigate whether long-term CsA therapy is effective and safe for treating AD.
Methods: We performed a retrospective study of the patients with AD and who were treated with CsA at Kyung Hee Medical Center between January 2001 and February 2008. Among 147 patients, 61 received CsA treatment for more than 6 months. To evaluate the efficacy of CsA treatment, the objective SCORAD was checked for all 61 patients at every visit. Extensive laboratory tests were performed every two months to assess the safety of treatment.
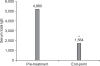
Results: The mean duration of CsA treatment was 13.5+/-8.4 months and the mean initial dose of CsA was 2.7+/-0.9 mg/kg/day. The mean objective SCORAD values significantly decreased from 34.1+/-11.2 at baseline to 11.4+/-10.7 after 6-month of CsA treatment (p<0.05). A significant decline of the SCORAD score was observed starting from 1-month of CsA treatment. The mean duration of remission was 4.5+/-2.9 months. A total of 13 adverse events in 10 patients were recorded during the study period. One patient dropped out due to renal dysfunction. Elevation of peripheral blood pressure was noted in 8 patients. Three patients complained of gastrointestinal troubles, and one patient had hypertrichosis, but the problems of these 4 patients were mild and easily treated.
Conclusion: We suggest that long-term, low-dose CsA treatment is safe and effective for patients who suffer from AD.
Keywords: Atopic dermatitis; Cyclosporine; Long-term treatment.
Figures




References
-
- James WD, Berger TG, Elston DM, Odom RB. Andrews' diseases of the skin: clinical dermatology. 10th ed. Philadelphia: Saunders Elsevier; 2006. pp. 69–77.
-
- Borel JF, Feurer C, Gubler HU, Stahelin H. Biological effects of cyclosporin A: a new antilymphocytic agent. Agents Actions. 1976;6:468–475. - PubMed
-
- Mueller W, Herrmann B. Cyclosporin A for psoriasis. N Engl J Med. 1979;301:555. - PubMed
-
- Nousari CH, Anhalt GJ. Immunosuppressive and immunomodulatory drugs. In: Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, editors. Fitzpatrick's dermatology in general medicine. 7th ed. New York: McGraw-Hill; 2008. pp. 2217–2223.
-
- Lee CS, Koo JYM. Cyclosporine. In: Wolverton SE, editor. Comprehensive dermatologic drug therapy. 2nd ed. Indiana: Saunders Elsevier; 2007. pp. 219–238.
LinkOut - more resources
Full Text Sources

