Stimulation of the extracellular matrix production in dermal fibroblasts by velvet antler extract
- PMID: 20548908
- PMCID: PMC2883420
- DOI: 10.5021/ad.2010.22.2.173
Stimulation of the extracellular matrix production in dermal fibroblasts by velvet antler extract
Abstract
Background: Fibroblasts produce many components of the extracellular matrix (ECM) and so they contribute to the maintenance of connective tissue integrity.
Objective: The aim of this study is to evaluate the effect of velvet antler extract (VAE) on the ECM production of dermal fibroblasts cultured in vitro.
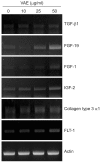
Methods: Primary cultured human dermal fibroblasts were treated with VAE, and then the ECM production was determined by RT-PCR, ELISA and Western blot analysis. Furthermore, the change of gene expression according to VAE treatment was evaluated by cDNA microarray.
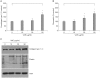
Results: VAE accelerated the growth of fibroblasts in a dose-dependent manner. VAE increased the production of several ECM components, including type 1 collagen, fibronectin and elastin. In line with these results, the phosphorylations of p42/44 ERK and p38 mitogen-activated protein kinase were markedly increased by VAE, suggesting that the enhancement of ECM production may be linked to the activation of intracellular signaling cascades. VAE also significantly increased cell migration on an in vitro scratch wound test. In cDNA microarray, many genes related with connective tissue integrity were identified to be up-regulated by VAE.
Conclusion: These results suggest that VAE has a potential to stimulate ECM production, and VAE may be applicable for maintaining the skin's texture.
Keywords: Extracellular matrix; Fibroblast; Velvet antler extract; cDNA microarray.
Figures



Similar articles
-
A Novel Compound Rasatiol Isolated from Raphanus sativus Has a Potential to Enhance Extracellular Matrix Synthesis in Dermal Fibroblasts.Ann Dermatol. 2013 Aug;25(3):315-20. doi: 10.5021/ad.2013.25.3.315. Epub 2013 Aug 13. Ann Dermatol. 2013. PMID: 24003274 Free PMC article.
-
Cedrol Enhances Extracellular Matrix Production in Dermal Fibroblasts in a MAPK-Dependent Manner.Ann Dermatol. 2012 Feb;24(1):16-21. doi: 10.5021/ad.2012.24.1.16. Epub 2012 Feb 2. Ann Dermatol. 2012. PMID: 22363150 Free PMC article.
-
Adiponectin Is Involved in Connective Tissue Growth Factor-Induced Proliferation, Migration and Overproduction of the Extracellular Matrix in Keloid Fibroblasts.Int J Mol Sci. 2017 May 12;18(5):1044. doi: 10.3390/ijms18051044. Int J Mol Sci. 2017. PMID: 28498357 Free PMC article.
-
Fibroblast extracellular matrix gene expression in response to keratinocyte-releasable stratifin.J Cell Biochem. 2006 May 15;98(2):383-93. doi: 10.1002/jcb.20782. J Cell Biochem. 2006. PMID: 16440305
-
Extracellular Matrix and Dermal Fibroblast Function in the Healing Wound.Adv Wound Care (New Rochelle). 2016 Mar 1;5(3):119-136. doi: 10.1089/wound.2014.0561. Adv Wound Care (New Rochelle). 2016. PMID: 26989578 Free PMC article. Review.
Cited by
-
Increment of growth factors in mouse skin treated with non-thermal plasma.Int J Med Sci. 2018 Jul 30;15(11):1203-1209. doi: 10.7150/ijms.26342. eCollection 2018. Int J Med Sci. 2018. PMID: 30123058 Free PMC article.
-
Combination of red ginseng and velvet antler extracts prevents skin damage by enhancing the antioxidant defense system and inhibiting MAPK/AP-1/NF-κB and caspase signaling pathways in UVB-irradiated HaCaT keratinocytes and SKH-1 hairless mice.J Ginseng Res. 2024 May;48(3):323-332. doi: 10.1016/j.jgr.2024.01.003. Epub 2024 Jan 17. J Ginseng Res. 2024. PMID: 38707646 Free PMC article.
-
Reduced Expression of YAP in Dermal Fibroblasts is Associated with Impaired Wound Healing in Type 2 Diabetic Mice.Tissue Eng Regen Med. 2017 Jan 17;14(1):49-55. doi: 10.1007/s13770-016-0019-9. eCollection 2017 Feb. Tissue Eng Regen Med. 2017. PMID: 30603461 Free PMC article.
-
Substance P modulates properties of normal and diabetic dermal fibroblasts.Tissue Eng Regen Med. 2016 Apr 5;13(2):155-161. doi: 10.1007/s13770-016-9085-2. eCollection 2016 Apr. Tissue Eng Regen Med. 2016. PMID: 30603395 Free PMC article.
-
Transcriptomic and in vivo approaches introduced human iPSC-derived microvesicles for skin rejuvenation.Sci Rep. 2023 Jun 20;13(1):9963. doi: 10.1038/s41598-023-36162-9. Sci Rep. 2023. PMID: 37339980 Free PMC article.
References
-
- Pieraggi MT, Bouissou H, Angelier C, Uhart D, Magnol JP, Kokolo J. The fibroblast. Ann Pathol. 1985;5:65–76. - PubMed
-
- Uitto J. Connective tissue biochemistry of the aging dermis. Age-related alterations in collagen and elastin. Dermatol Clin. 1986;4:433–446. - PubMed
-
- Ignotz RA, Massague J. Transforming growth factor-beta stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J Biol Chem. 1986;261:4337–4345. - PubMed
-
- Jhon GJ, Park SY, Han SY, Lee S, Kim Y, Chang YS. Studies of the chemical structure of gangliosides in deer antler, Cervus nippon. Chem Pharm Bull (Tokyo) 1999;47:123–127. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

