A crucial role for infected-cell/antibody immune complexes in the enhancement of endogenous antiviral immunity by short passive immunotherapy
- PMID: 20548955
- PMCID: PMC2883599
- DOI: 10.1371/journal.ppat.1000948
A crucial role for infected-cell/antibody immune complexes in the enhancement of endogenous antiviral immunity by short passive immunotherapy
Abstract
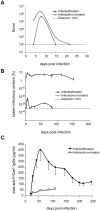
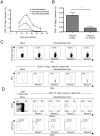
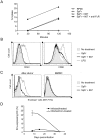
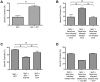
Antiviral monoclonal antibodies (mAbs) represent promising therapeutics. However, most mAbs-based immunotherapies conducted so far have only considered the blunting of viral propagation and not other possible therapeutic effects independent of virus neutralization, namely the modulation of the endogenous immune response. As induction of long-term antiviral immunity still remains a paramount challenge for treating chronic infections, we have asked here whether neutralizing mAbs can, in addition to blunting viral propagation, exert immunomodulatory effects with protective outcomes. Supporting this idea, we report here that mice infected with the FrCas(E) murine retrovirus on day 8 after birth die of leukemia within 4-5 months and mount a non-protective immune response, whereas those rapidly subjected to short immunotherapy with a neutralizing mAb survive healthy and mount a long-lasting protective antiviral immunity with strong humoral and cellular immune responses. Interestingly, the administered mAb mediates lysis of infected cells through an antibody-dependent cell cytotoxicity (ADCC) mechanism. In addition, it forms immune complexes (ICs) with infected cells that enhance antiviral CTL responses through Fc gammaR-mediated binding to dendritic cells (DCs). Importantly, the endogenous antiviral antibodies generated in mAb-treated mice also display the same properties, allowing containment of viral propagation and enhancement of memory cellular responses after disappearance of the administered mAb. Thus, our data demonstrate that neutralizing antiviral mAbs can act as immunomodulatory agents capable of stimulating a protective immunity lasting long after the end of the treatment. They also show an important role of infected-cells/antibody complexes in the induction and the maintenance of protective immunity through enhancement of both primary and memory antiviral T-cell responses. They also indicate that targeting infected cells, and not just viruses, by antibodies can be crucial for elicitation of efficient, long-lasting antiviral T-cell responses. This must be considered when designing antiviral mAb-based immunotherapies.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
Long-lasting protective antiviral immunity induced by passive immunotherapies requires both neutralizing and effector functions of the administered monoclonal antibody.J Virol. 2010 Oct;84(19):10169-81. doi: 10.1128/JVI.00568-10. Epub 2010 Jul 7. J Virol. 2010. PMID: 20610721 Free PMC article.
-
Endogenous cytotoxic T-cell response contributes to the long-term antiretroviral protection induced by a short period of antibody-based immunotherapy of neonatally infected mice.J Virol. 2008 Feb;82(3):1339-49. doi: 10.1128/JVI.01970-07. Epub 2007 Nov 21. J Virol. 2008. PMID: 18032505 Free PMC article.
-
Efficient mother-to-child transfer of antiretroviral immunity in the context of preclinical monoclonal antibody-based immunotherapy.J Virol. 2006 Oct;80(20):10191-200. doi: 10.1128/JVI.01095-06. J Virol. 2006. PMID: 17005696 Free PMC article.
-
Converting monoclonal antibody-based immunotherapies from passive to active: bringing immune complexes into play.Emerg Microbes Infect. 2016 Aug 17;5(8):e92. doi: 10.1038/emi.2016.97. Emerg Microbes Infect. 2016. PMID: 27530750 Free PMC article. Review.
-
Antiviral Monoclonal Antibodies: Can They Be More Than Simple Neutralizing Agents?Trends Microbiol. 2015 Oct;23(10):653-665. doi: 10.1016/j.tim.2015.07.005. Trends Microbiol. 2015. PMID: 26433697 Free PMC article. Review.
Cited by
-
Monoclonal antibodies for prophylactic and therapeutic use against viral infections.Pediatr Pol. 2013 Sep-Oct;88(5):T15-T23. doi: 10.1016/j.pepo.2013.08.006. Epub 2013 Aug 23. Pediatr Pol. 2013. PMID: 32287402 Free PMC article. Review.
-
Anti-HERV-K (HML-2) capsid antibody responses in HIV elite controllers.Retrovirology. 2017 Aug 22;14(1):41. doi: 10.1186/s12977-017-0365-2. Retrovirology. 2017. PMID: 28830571 Free PMC article.
-
Blocking α4β7 integrin delays viral rebound in SHIVSF162P3-infected macaques treated with anti-HIV broadly neutralizing antibodies.Sci Transl Med. 2021 Aug 18;13(607):eabf7201. doi: 10.1126/scitranslmed.abf7201. Sci Transl Med. 2021. PMID: 34408080 Free PMC article.
-
Immunoregulatory functions of immune complexes in vaccine and therapy.EMBO Mol Med. 2016 Oct 4;8(10):1120-1133. doi: 10.15252/emmm.201606593. Print 2016 Oct. EMBO Mol Med. 2016. PMID: 27572622 Free PMC article. Review.
-
Poorly neutralizing cross-reactive antibodies against the fusion loop of West Nile virus envelope protein protect in vivo via Fcgamma receptor and complement-dependent effector mechanisms.J Virol. 2011 Nov;85(22):11567-80. doi: 10.1128/JVI.05859-11. Epub 2011 Sep 14. J Virol. 2011. PMID: 21917960 Free PMC article.
References
-
- Takada A, Ebihara H, Jones S, Feldmann H, Kawaoka Y. Protective efficacy of neutralizing antibodies against Ebola virus infection. Vaccine. 2007;25:993–999. - PubMed
-
- Hangartner L, Zinkernagel RM, Hengartner H. Antiviral antibody responses: the two extremes of a wide spectrum. Nat Rev Immunol. 2006;6:231–243. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

