Oxidized LDL up-regulates the ascorbic acid transporter SVCT2 in endothelial cells
- PMID: 20549544
- PMCID: PMC3725123
- DOI: 10.1007/s11010-010-0516-4
Oxidized LDL up-regulates the ascorbic acid transporter SVCT2 in endothelial cells
Abstract
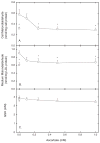
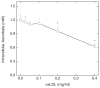
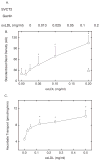
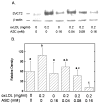
Endothelial dysfunction is an early manifestation of atherosclerosis caused in part by oxidized LDL (oxLDL). Since vitamin C, or ascorbic acid, prevents several aspects of endothelial dysfunction, the effects of oxLDL on oxidative stress and regulation of the ascorbate transporter, SVCT2, were studied in cultured EA.hy926 endothelial cells. Cells cultured for 18 h with 0.2 mg/ml oxLDL showed increased lipid peroxidation that was prevented by a single addition of 0.25 mM ascorbate at the beginning of the incubation. This protection caused a decrease in intracellular ascorbate, but no change in the cell content of GSH. In the absence of ascorbate, oxLDL increased SVCT2 protein and function during 18 h in culture. Although culture of the cells with ascorbate did not affect SVCT2 protein expression, the oxLDL-induced increase in SVCT2 protein expression was prevented by ascorbate. These results suggest that up-regulation of endothelial cell SVCT2 expression and function may help to maintain intracellular ascorbate during oxLDL-induced oxidative stress, and that ascorbate in turn can prevent this effect.
Figures
References
-
- Steinberg D, Parthasarathy S, Carew TE, et al. Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med. 1989;320:915–924. - PubMed
-
- Steinbrecher UP. Role of superoxide in endothelial-cell modification of low-density lipoproteins. Biochim Biophys Acta. 1988;959:20–30. - PubMed
-
- Martin A, Frei B. Both intracellular and extracellular vitamin C inhibit atherogenic modification of LDL by human vascular endothelial cells. Arterioscler Thromb Vasc Biol. 1997;17:1583–1590. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

