Origin, maturation, and astroglial transformation of secondary radial glial cells in the developing dentate gyrus
- PMID: 20549747
- PMCID: PMC2946789
- DOI: 10.1002/glia.21029
Origin, maturation, and astroglial transformation of secondary radial glial cells in the developing dentate gyrus
Abstract
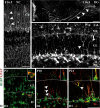
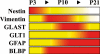
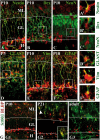
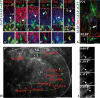
The dentate gyrus is a brain region where neurons are continuously born throughout life. In the adult, the role of its radial glia in neurogenesis has attracted much attention over the past years; however, little is known about the generation and differentiation of glial cells and their relationship to radial glia during the ontogenetic development of this brain structure. Here, we combine immunohistochemical phenotyping using antibodies against glial marker proteins with BrdU birthdating to characterize the development of the secondary radial glial scaffold in the dentate gyrus and its potential to differentiate into astrocytes. We demonstrate that the expression of brain lipid-binding protein, GLAST, and glial fibrillary acidic protein (GFAP) characterizes immature differentiating cells confined to an astrocytic fate in the early postnatal dentate gyrus. On the basis of our studies, we propose a model where immature astrocytes migrate radially through the granule cell layer to adopt their final positions in the molecular layer of the dentate gyrus. Time-lapse imaging of acute hippocampal slices from hGFAP-eGFP transgenic mice provides direct evidence for such a migration mode of differentiating astroglial cells in the developing dentate gyrus.
(c) 2010 Wiley-Liss, Inc.
Figures







References
-
- Altman J, Bayer SA. Migration and distribution of two populations of hippocampal granule cell precursors during the perinatal and postnatal periods. J Comp Neurol. 1990a;301(3):365–81. - PubMed
-
- Altman J, Bayer SA. Mosaic organization of the hippocampal neuroepithelium and the multiple germinal sources of dentate granule cells. J Comp Neurol. 1990b;301(3):325–42. - PubMed
-
- Alves JA, Barone P, Engelender S, Froes MM, Menezes JR. Initial stages of radial glia astrocytic transformation in the early postnatal anterior subventricular zone. J Neurobiol. 2002;52(3):251–65. - PubMed
-
- Barry D, McDermott K. Differentiation of radial glia from radial precursor cells and transformation into astrocytes in the developing rat spinal cord. Glia. 2005;50(3):187–97. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

