A statistical mechanical theory for a two-dimensional model of water
- PMID: 20550408
- PMCID: PMC2902533
- DOI: 10.1063/1.3454193
A statistical mechanical theory for a two-dimensional model of water
Abstract
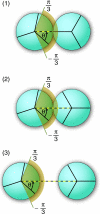
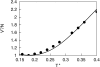
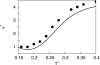
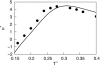
We develop a statistical mechanical model for the thermal and volumetric properties of waterlike fluids. Each water molecule is a two-dimensional disk with three hydrogen-bonding arms. Each water interacts with neighboring waters through a van der Waals interaction and an orientation-dependent hydrogen-bonding interaction. This model, which is largely analytical, is a variant of the Truskett and Dill (TD) treatment of the "Mercedes-Benz" (MB) model. The present model gives better predictions than TD for hydrogen-bond populations in liquid water by distinguishing strong cooperative hydrogen bonds from weaker ones. We explore properties versus temperature T and pressure p. We find that the volumetric and thermal properties follow the same trends with T as real water and are in good general agreement with Monte Carlo simulations of MB water, including the density anomaly, the minimum in the isothermal compressibility, and the decreased number of hydrogen bonds for increasing temperature. The model reproduces that pressure squeezes out water's heat capacity and leads to a negative thermal expansion coefficient at low temperatures. In terms of water structuring, the variance in hydrogen-bonding angles increases with both T and p, while the variance in water density increases with T but decreases with p. Hydrogen bonding is an energy storage mechanism that leads to water's large heat capacity (for its size) and to the fragility in its cagelike structures, which are easily melted by temperature and pressure to a more van der Waals-like liquid state.
Figures




Similar articles
-
Simple model of hydrophobic hydration.J Phys Chem B. 2012 May 31;116(21):6177-86. doi: 10.1021/jp300743a. Epub 2012 May 21. J Phys Chem B. 2012. PMID: 22564051 Free PMC article.
-
Analytical model for three-dimensional Mercedes-Benz water molecules.Phys Rev E Stat Nonlin Soft Matter Phys. 2012 Jun;85(6 Pt 1):061503. doi: 10.1103/PhysRevE.85.061503. Epub 2012 Jun 25. Phys Rev E Stat Nonlin Soft Matter Phys. 2012. PMID: 23005100 Free PMC article.
-
Liquid-liquid critical point in a simple analytical model of water.Phys Rev E. 2016 Oct;94(4-1):042126. doi: 10.1103/PhysRevE.94.042126. Epub 2016 Oct 21. Phys Rev E. 2016. PMID: 27841542
-
Modeling water, the hydrophobic effect, and ion solvation.Annu Rev Biophys Biomol Struct. 2005;34:173-99. doi: 10.1146/annurev.biophys.34.040204.144517. Annu Rev Biophys Biomol Struct. 2005. PMID: 15869376 Review.
-
Recent developments in the theoretical, simulational, and experimental studies of the role of water hydrogen bonding in hydrophobic phenomena.Adv Colloid Interface Sci. 2016 Sep;235:23-45. doi: 10.1016/j.cis.2016.05.006. Epub 2016 May 18. Adv Colloid Interface Sci. 2016. PMID: 27312562 Review.
Cited by
-
Modelling water with simple Mercedes-Benz models.Mol Simul. 2019;45(4-5):279-294. doi: 10.1080/08927022.2018.1502430. Epub 2018 Aug 1. Mol Simul. 2019. PMID: 31156291 Free PMC article.
-
Simple model of hydrophobic hydration.J Phys Chem B. 2012 May 31;116(21):6177-86. doi: 10.1021/jp300743a. Epub 2012 May 21. J Phys Chem B. 2012. PMID: 22564051 Free PMC article.
-
Analytical 2-Dimensional Model of Nonpolar and Ionic Solvation in Water.J Phys Chem B. 2021 Feb 25;125(7):1861-1873. doi: 10.1021/acs.jpcb.0c10329. Epub 2021 Feb 4. J Phys Chem B. 2021. PMID: 33539097 Free PMC article.
-
How Water's Properties Are Encoded in Its Molecular Structure and Energies.Chem Rev. 2017 Oct 11;117(19):12385-12414. doi: 10.1021/acs.chemrev.7b00259. Epub 2017 Sep 26. Chem Rev. 2017. PMID: 28949513 Free PMC article.
-
Statistical-mechanical liquid theories reproduce anomalous thermodynamic properties of explicit two-dimensional water models.Phys Rev E. 2022 Sep;106(3-1):034115. doi: 10.1103/PhysRevE.106.034115. Phys Rev E. 2022. PMID: 36266898 Free PMC article.
References
-
- Röntgen W. C., Ann. Phys. Chem. ANPYA2 45, 91 (1892).10.1002/andp.18922810108 - DOI
-
- Pople J. A., Proc. R. Soc. London, Ser. A PRLAAZ 205, 163 (1951).10.1098/rspa.1951.0024 - DOI
-
- Bell G. M., J. Phys. C JPSOAW 5, 889 (1972).10.1088/0022-3719/5/9/004 - DOI
-
- Rahman A. and Stillinger F. H., J. Chem. Phys. JCPSA6 57, 4009 (1972).10.1063/1.1678874 - DOI
-
- Borick S. S., Debenedetti P. G., and Sastry S., J. Phys. Chem. JPCHAX 99, 3781 (1995).10.1021/j100011a054 - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

