Human polyomavirus JC (JCV) infection of human B lymphocytes: a possible mechanism for JCV transmigration across the blood-brain barrier
- PMID: 20550458
- PMCID: PMC2928669
- DOI: 10.1086/653823
Human polyomavirus JC (JCV) infection of human B lymphocytes: a possible mechanism for JCV transmigration across the blood-brain barrier
Abstract
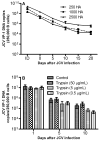
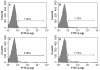
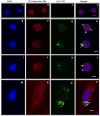
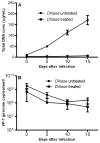
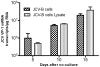
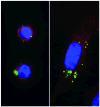
It has been suggested that JC virus (JCV) might travel to the central nervous system in infected B cells. Moreover, recent data suggest the presence of JCV in bone marrow plasma cells. However, the evidence for infection and replication of JCV in B cells is unclear. To address this question, we infected Epstein-Barr virus-transformed B cells with JCV and found that the viral genome decreased >1000-fold from days 0 to 20 after infection, which concurred with the absence of viral early and late messenger RNA transcripts and proteins. However, immunofluorescent images of B cells infected with fluorescein isothiocyanate-conjugated JCV demonstrated that JCV enters the B cells, and DNase protection assay confirmed the presence of intact JCV virions inside the B cells. Moreover, JCV-infected B cells were able to transmit infection to naive glial cells. These data confirm that JCV nonproductively infects B cells and possibly uses them as a vehicle for transmigration across the blood-brain barrier.
Conflict of interest statement
Potential conflicts of interest: None
Figures
Comment in
-
The curious incident of the dog in the nighttime: does the absence of virus replication in Epstein-Barr virus-transformed B cells point to an important feature of JC virus biology?J Infect Dis. 2010 Jul 15;202(2):181-3. doi: 10.1086/653824. J Infect Dis. 2010. PMID: 20550457 No abstract available.
References
-
- Astrom KE, Mancall EL, Richardson EP., Jr Progressive multifocal leuko-encephalopathy; a hitherto unrecognized complication of chronic lymphatic leukaemia and Hodgkin's disease. Brain. 1958;81:93–111. - PubMed
-
- Frisque RJ, White FA., III . The molecular biology of JC virus, causative agent of progressive multifocal leukoencephalopathy. In: R PR, editor. Molecular Neurovirology. Totowa, NJ: Humana Press; 1992. pp. 25–158.
-
- Padgett BL, Walker DL, ZuRhein GM, Eckroade RJ, Dessel BH. Cultivation of papova-like virus from human brain with progressive multifocal leucoencephalopathy. Lancet. 1971;1:1257–60. - PubMed
-
- Richardson EP., Jr Our evolving understanding of progressive multifocal leukoencephalopathy. Ann N Y Acad Sci. 1974;230:358–64. - PubMed
-
- Doerries K. Human Polyomavirus JC and BK Persistent Infection. In: Ahsan N, editor. Polyomaviruses and Human Diseases. Vol. 577. Springer Science and Landes Bioscience; 2006. pp. 102–116. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

