Immunoglobulin G fragment C receptor polymorphisms and KRAS mutations: are they useful biomarkers of clinical outcome in advanced colorectal cancer treated with anti-EGFR-based therapy?
- PMID: 20550522
- PMCID: PMC11158139
- DOI: 10.1111/j.1349-7006.2010.01621.x
Immunoglobulin G fragment C receptor polymorphisms and KRAS mutations: are they useful biomarkers of clinical outcome in advanced colorectal cancer treated with anti-EGFR-based therapy?
Abstract
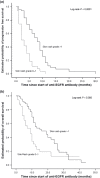
KRAS mutations have been identified as a strong predictor of resistance to anti-epidermal growth factor receptor (EGFR) therapies. Besides inhibiting the EGFR pathway, anti-EGFR monoclonal antibodies may exert antitumor effects through antibody-dependent cell-mediated cytotoxicity (ADCC). Through this mechanism, the antibody fragment C portion (Fcγ) interacts with Fc receptors (FcγRs) expressed by immune effectors cells. We investigated the association of FcγR polymorphisms and KRAS mutation with the clinical outcome of 104 refractory metastatic colorectal cancer (mCRC) patients treated with anti-EGFR antibodies. FcγRIIa-H131R and FcγRIIIa-V158F polymorphisms were analyzed in genomic DNA using a 48.48 dynamic array on the BioMark system (Fluidigm, South Sanfrancisco, CA, USA). Tumor tissues from 96 cases were screened for KRAS mutations. KRAS mutation was associated with a lower response rate (RR) (P = 0.035) and a shorter progression-free survival (PFS) (3 vs 7 months; P = 0.36). FcγRIIa-H131R and FcγRIIIa-V158F polymorphisms did not show statistically significant associations with response, PFS, or KRAS status. In the logistic regression analysis, KRAS status (P = 0.04) and skin toxicity (P = 0.03) were associated with RR. By multivariate analysis, the clinical risk classification (P = 0.006) and skin toxicity (P < 0.0001) were found to be independent risk factors for PFS. In conclusion, the FcγRIIa and FcγRIIIa polymorphisms are not useful as molecular markers for clinical outcome in mCRC patients. To date, the EORTC (European Organization for Research and Treatment of Cancer Classification), skin toxicity, and KRAS status are the only reliable biomarkers to identify patients that would benefit from anti-EGFR therapy.
© 2010 Japanese Cancer Association.
Figures
Similar articles
-
Impact of Fc{gamma}RIIa-Fc{gamma}RIIIa polymorphisms and KRAS mutations on the clinical outcome of patients with metastatic colorectal cancer treated with cetuximab plus irinotecan.J Clin Oncol. 2009 Mar 1;27(7):1122-9. doi: 10.1200/JCO.2008.18.0463. Epub 2009 Jan 21. J Clin Oncol. 2009. PMID: 19164213
-
Genetic polymorphisms of FcγRIIa and FcγRIIIa are not predictive of clinical outcomes after cetuximab plus irinotecan chemotherapy in patients with metastatic colorectal cancer.Oncology. 2012;82(2):83-9. doi: 10.1159/000335959. Epub 2012 Feb 8. Oncology. 2012. PMID: 22327884
-
Clinical usefulness of KRAS, BRAF, and PIK3CA mutations as predictive markers of cetuximab efficacy in irinotecan- and oxaliplatin-refractory Japanese patients with metastatic colorectal cancer.Int J Clin Oncol. 2013 Aug;18(4):670-7. doi: 10.1007/s10147-012-0422-8. Epub 2012 May 26. Int J Clin Oncol. 2013. PMID: 22638623
-
Recommendations from the EGAPP Working Group: can testing of tumor tissue for mutations in EGFR pathway downstream effector genes in patients with metastatic colorectal cancer improve health outcomes by guiding decisions regarding anti-EGFR therapy?Genet Med. 2013 Jul;15(7):517-27. doi: 10.1038/gim.2012.184. Epub 2013 Feb 21. Genet Med. 2013. PMID: 23429431
-
Progress in metastatic colorectal cancer: growing role of cetuximab to optimize clinical outcome.Clin Transl Oncol. 2010 Aug;12(8):533-42. doi: 10.1007/s12094-010-0551-3. Clin Transl Oncol. 2010. PMID: 20709651 Review.
Cited by
-
Impact of KRAS mutation on the tumor microenvironment in colorectal cancer.Int J Biol Sci. 2024 Mar 3;20(5):1947-1964. doi: 10.7150/ijbs.88779. eCollection 2024. Int J Biol Sci. 2024. PMID: 38481800 Free PMC article. Review.
-
The predictive role of skin rash with cetuximab and panitumumab in colorectal cancer patients: a systematic review and meta-analysis of published trials.Target Oncol. 2013 Sep;8(3):173-181. doi: 10.1007/s11523-013-0257-x. Epub 2013 Jan 16. Target Oncol. 2013. PMID: 23321777
-
Anti-GD2 mAbs and next-generation mAb-based agents for cancer therapy.Immunotherapy. 2016 Sep;8(9):1097-117. doi: 10.2217/imt-2016-0021. Immunotherapy. 2016. PMID: 27485082 Free PMC article. Review.
-
The global landscape of approved antibody therapies.Antib Ther. 2022 Sep 6;5(4):233-257. doi: 10.1093/abt/tbac021. eCollection 2022 Oct. Antib Ther. 2022. PMID: 36213257 Free PMC article. Review.
-
Combination of KIR2DS4 and FcγRIIa polymorphisms predicts the response to cetuximab in KRAS mutant metastatic colorectal cancer.Sci Rep. 2019 Feb 22;9(1):2589. doi: 10.1038/s41598-019-39291-2. Sci Rep. 2019. PMID: 30796344 Free PMC article. Clinical Trial.
References
-
- Cunningham D, Humblet Y, Siena S et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan‐refractory metastatic colorectal cancer. N Engl J Med 2004; 351: 337–45. - PubMed
-
- Saltz LB, Meropol NJ, Loehrer PJ Sr, Needle MN, Kopit J, Mayer RJ. Phase II trial of cetuximab in patients with refractory colorectal cancer that expresses the epidermal growth factor receptor. J Clin Oncol 2004; 22: 1201–8. - PubMed
-
- Van Cutsem E, Köhne CH, Hitre E et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med 2009; 360: 1408–17. - PubMed
-
- Van Cutsem E, Peeters M, Siena S et al. Open‐label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy‐refractory metastatic colorectal cancer. J Clin Oncol 2007; 25: 1658–64. - PubMed
-
- Amado RG, Wolf M, Peeters M et al. Wild‐type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol 2008; 26: 1626–34. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

